Abstract
Dieulafoy lesions, vascular anomalies typically found along the gastrointestinal tract, have been viewed as rare and obscure causes of sudden intestinal bleeding, especially in pediatric patients. Since their discovery in the late 19th century, the reported incidence has increased. This is due to an increased awareness of, and knowledge about, their presentation and to advanced endoscopic diagnosis and therapy. Our patient was a three-year-old male, without a complex medical history. He presented to the emergency department with acute hematemesis with blood clots and acute anemia requiring blood transfusion. Endoscopy revealed four isolated Dieulafoy lesions along the lesser curvature of the stomach, which were treated with an epinephrine injection. The Dieulafoy lesion, although thought to be rare, should be considered when investigating an acute gastrointestinal bleed. These lesions have been successfully treated endoscopically. Appropriate anticipation and preparation for diagnosis and therapy can lead to optimal outcomes for the pediatric patient.
Upper gastrointestinal (UGI) bleeding accounts for 10-15% of all admissions to pediatric gastroenterologists, and although uncommon in the pediatric population, presentation of UGI bleeding may span from anemia to hypovolemic shock. Therefore, identifying and understanding the differential diagnoses for pediatric UGI bleeds is essential [1]. Dieulafoy lesions are a rare source of GI bleeding in the pediatric population. We report a three-year-old male, presenting with hematemesis and melena, who was diagnosed endoscopically with multiple Dieulafoy lesions. He was successfully treated with epinephrine injection.
Our patient was a three-year-old previously healthy African American male, with history of intermittent asthma, eczema, and intermittent constipation. He presented to the emergency department with hematemesis and melena. He had no surgical history other than a circumcision in infancy. The only medications he took were Ibuprofen, approximately twice monthly, and an albuterol inhaler as needed. His mother denied any ingestions, chemicals or foreign bodies, or previous history of hematemesis. There were no known significant GI conditions within the family.
On the morning of admission, the patient woke up with diffuse abdominal pain and subsequent emesis containing multiple blood clots. Upon arrival, his weight and height were 15 kg and 94 cm, respectively. His vital signs were within normal limits for age, and he was afebrile. Upon physical exam, he was in no acute distress, with moist mucus membranes, no respiratory distress or cardiac abnormalities. He did not have any abnormal skin findings or signs of bleeding. His initial laboratory studies included white blood cell count of 11.1×103/µL, hemoglobin 9.6 g/dL, hematocrit 27.6%, mean corpuscular volume 78.1 fL, platelets 307×103/µL, prothrombin time 13.9, international normalized ratio 1.07, partial thromboplastin time 27.5.
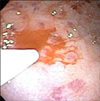
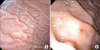
Therapeutic endoscopy was performed in an endoscopy unit in a free standing children's hospital (Johns Hopkins All Children's Hospital) with anesthesiologist administered intravenous propofol. Endoscopy revealed clotted blood and debris consistent with recent bleeding. Inspection of the stomach body revealed four individual lesions that appeared to be Dieulafoy in origin. The lesions were injected with 1:10,000 epinephrine; the first identified lesion was injected with 2 mL, and subsequent lesions with 0.1 to 0.3 mL aliquots (Fig. 1, 2, 3). Elective endo-clipping was not performed at that session due to adequate hemostasis.
Later that evening, he had a subsequent episode of hematemesis with a low-grade fever. His hemoglobin decreased to 6.9 mg/dL, and he received a packed red blood cell transfusion. Repeat endoscopy showed no active bleeding. The four previously injected lesions were easily visualized; therefore, the endoscopist did not provide further hemostatic therapy. Hematemesis was felt to be related to old blood. The patient was discharged home and prescribed sucralfate every 8 hours, ranitidine twice daily, and lactulose as needed for constipation.
He was seen in clinic two weeks after discharge and appeared to be doing well with no further complaints of nausea, vomiting, or abdominal pain. The patient subsequently had recurrent non-bloody emesis four months after his hospitalization with simultaneous fecal retention and constipation. The patient underwent a bowel clean out with lactulose and esophagogastroduodenoscopy was negative for GI bleeding.
The differential diagnosis for UGI bleeding is vast and dependent upon the age, co-morbidities, and overall health of the patient. More than 280 cases have been reported since Paul Dieulafoy initially described these lesions more than 100 years ago [2], and 24 pediatric cases have been published in English, accessed by PubMed and Medline, from 1990 to 2011 [3].
The Dieulafoy lesion is a normally structured vessel with an unexpectedly large diameter, 1-3 mm wide, and protruding into the mucosa from the submucosa. This protrusion creates a small wall defect with fibrinoid necrosis found at the base [24]. Seventy percent of the lesions are found in the stomach, usually along the lesser curvature, with more than 80-95% found within 6 cm of the gastro-esophageal junction [2].
There have been multiple proposed mechanisms to describe the pathologic bleeding of Dieulafoy lesions including congenital defects that develop acute ruptures, as well as the arterial nature of the vessel and expected pulsatile movement eroding the mucosa over time, and subsequent rupture [4567].
Dieulafoy lesions have been reported in all age groups including a small number of cases in newborns, suggesting these can be congenital. The age of the patient influences the type of predisposition or risk factors for bleeding as well as treatment and management. They are found more commonly in adults with co-morbidities, including exposure to aspirin, NSAIDs and warfarin, although no causal relationship has been proven. They are found in men twice as frequently as women [2]; however, the pediatric population does not appear to have a gender preference [3].
Dieulafoy lesions are rarely reported in the pediatric population. According to Senger and Kanthan [3], 24 cases of pediatric Dieulafoy lesions have been reported, from 1990 to 2011, with known age ranges from 8 weeks to 18 years, mean age 10 years old. In the pediatric population, 10 lesions have been found in the stomach, 2 in the duodenum, 5 in the jejunum, and 5 in the ileum, and a single lesion found each in the sigmoid, anorectal junction, and anal canal.
However, Dieulafoy lesions are believed to be underreported. The low reported incidence may be due to lack of awareness, abrupt presentation, or inability to visualize the lesion for accurate diagnosis via endoscopy [2]. Endoscopies may not reveal a diagnosis due to excessive bleeding or because lesions may be very subtle. Criteria to diagnose a Dieulafoy lesion are defined as the lesion having: (1) active arterial “spurting” or “oozing” from a small defect in the mucosa (<3 mm); (2) a visible, protruding vessel from a normal or slight defect in mucosa; and/or (3) a fresh blood clot adhering to the defect of normal mucosa [89]. Certain cases have been diagnosed with Technetium-99m labeled red blood cell scanning, angiography, or computed tomography angiography when the source of the bleed cannot be visualized [2].
Surgical management was the only treatment initially available, and it was associated with significant morbidity and death. The introduction and evolution of endoscopy for the diagnosis and treatment of Dieulafoy lesions have reduced the need for more invasive surgery [2]. Approximately 70% of initial endoscopies can detect a lesion if there is active bleeding of at least 0.5 mL/min [10]. As more Dieulafoy lesions have been discovered via endoscopy alone or with adjuvant interventions, clinicians and endoscopists are increasingly including these lesions in the differential diagnosis of a GI bleed [2].
Although initial endoscopy may be therapeutic, many patients with Dieulafoy lesions may require multiple procedures for diagnosis and/or cessation of bleeding. Advanced techniques such as regional injections (epinephrine or sclerotherapy), mechanical interventions (banding, hemoclips), and thermal treatments (heat probe coagulation, argon plasma coagulation and electrocoagulation) have been overall effective, even in the pediatric population, with individually varying success. A combination of these therapies has shown to be more effective than monotherapy. A practitioner may consider angiography and embolization for treatment, but these therapies are typically reserved for lesions located in the bronchi, lower GI tract, or upper GI lesions refractive to other therapies [2].
Endoscopic treatment for Dieulafoy lesions is gaining traction in the pediatric population. In Senger and Kanthan's literature review [3], twelve pediatric patients were treated surgically, and fourteen were treated endoscopically (via injection, banding, or thermocoagulation). Unlike the adult population, therapeutic protocols for GI bleeds are limited, as optimal diagnostic and therapeutic treatment options for children have yet to be determined [11]. There is limited evidence regarding the safety and efficacy of therapeutic techniques to manage pediatric upper GI bleeds. However, several therapies used in adults are safely used in pediatric patients, including epinephrine injection therapy and hemoclip application.
Therapeutic endoscopy is recommended as first-line management for patients who are actively bleeding or for patients at risk for emergent surgery, recurrent bleeding, or mortality. Primary hemostasis is best accomplished with hemoclip application rather than an injection or thermocoagulation techniques used in adults. Hemoclipping was 95% successful when treating a Dieulafoy lesion [112]. Clip application can be limited if the lesion is difficult to access due to location, i.e. lesser curvature (most common site for Dieulafoy lesions), the lesions are large, i.e. >30 mm, or beyond the clip's width. In these cases, other modalities may be more suitable [13]. Fortunately, for our patient, multiple epinephrine injections were sufficient to reach primary hemostasis without recurrent hematemesis or rebleeding.
As more pediatric cases of Dieulafoy lesions are reported, physicians will become increasingly aware of them as a potential cause of pediatric GI bleeding. It follows, therefore, that the publication of more pediatric cases and therapeutic techniques will enhance our understanding of the pathophysiology and the optimal management for upper GI bleeding, including Dieulafoy lesions, to eliminate recurrent bleeding, co-morbidities, and mortality. It is believed that with this growing body of information, Dieulafoy lesions will become a more common diagnosis following endoscopy, and management of pediatric GI bleeding will continue to improve.
Figures and Tables
References
1. Ünal F, Çakır M, Baran M, Duygulu Ş, Aydoğdu S. Application of endoscopic hemoclips for nonvariceal upper gastrointestinal bleeding in children. Turk J Gastroenterol. 2014; 25:147–151.

2. Baxter M, Aly EH. Dieulafoy's lesion: current trends in diagnosis and management. Ann R Coll Surg Engl. 2010; 92:548–554.

3. Senger JL, Kanthan R. The Evolution of Dieulafoy's Lesion Since 1897: then and now-a journey through the lens of a pediatric lesion with literature review. Gastroenterol Res Pract. 2012; 2012:432517.

5. Alshumrani G, Almuaikeel M. Angiographic findings and endovascular embolization in Dieulafoy disease: a case report and literature review. Diagn Interv Radiol. 2006; 12:151–154.
6. Löschhorn C, Nierhoff N, Mayer R, Zaunbauer W, Neuweiler J, Knoblauch A. Dieulafoy's disease of the lung: a potential disaster for the bronchoscopist. Respiration. 2006; 73:562–565.

7. Ajay G, Mohnish C. Anorectal Dieulafoy's lesion. Indian J Surg. 2006; 68:325–327.
8. Apiratpracha W, Ho JK, Powell JJ, Yoshida EM. Acute lower gastrointestinal bleeding from a dieulafoy lesion proximal to the anorectal junction post-orthotopic liver transplant. World J Gastroenterol. 2006; 12:7547–7548.

9. Dieulafoy G. Exulceratio simplex: lecons 1-3. In : Dieulafoy G, editor. Clinique medicale de l'hotel Dieu de Paris. Paris: Masson et Cie;1898. p. 1–38.
10. Driver CP, Bruce J. An unusual cause of massive gastric bleeding in a child. J Pediatr Surg. 1997; 32:1749–1750.

11. Arain Z, Rossi TM. Gastrointestinal bleeding in children: an overview of conditions requiring nonoperative management. Semin Pediatr Surg. 1999; 8:172–180.

12. Kapetanos D, Beltsis A, Chatzimavroudis G, Katsinelos P. The use of endoclips in the treatment of non-variceal gastrointestinal bleeding. Surg Laparosc Endosc Percutan Tech. 2009; 19:2–10.

13. British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. 2002; 51:Suppl 4. iv1–iv6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download