Abstract
Purpose
To study whether breastfeeding and breastfeeding status during gluten introduction influences the age at diagnosis of celiac disease (CD). In addition to study, whether the timing of gluten introduction influences the age at diagnosis of CD.
Methods
It was a hospital based observational study. Total 198 patients diagnosed with CD as per modified European Society of Pediatric Gastroenterology, Hepatology and Nutrition (2012) criteria, aged between 6 months to 6 years were included. Detail history taken with special emphasis on breastfeeding and age of gluten introduction. Standard statistical methods used to analyze the data.
Results
Mean±standard deviation age of onset and diagnosis of CD in breastfed cases was 2.81±1.42 years and 3.68 ±1.55 years respectively as compared to 1.84±1.36 years and 2.70±1.65 years respectively in not breastfed cases (p<0.05). Those who had continued breastfeeding during gluten introduction and of longer duration had significantly delayed onset of disease. The age at onset of CD was under one year in 40.42% of the cases, who had started gluten before 6 months of age compared to only 12.58% of those who had started gluten later (p<0.001). The proposed statistical model showed that two variables, i.e., breast feeding status during gluten introduction and age at gluten introduction positively influencing the age at diagnosis of CD.
Celiac disease (CD) is defined as an immune mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals, characterized by the presence of a variable combination of gluten-dependent clinical manifestations, a CD-specific antibodies, human leukocyte antigen (HLA)-DQ2 and HLA-DQ8 haplotypes, and enteropathy [1]. The CD is multifactorial in origin. The onset of the disease is an influence of genetic and environmental factors. The main environmental factor is gluten. About 90-95% of the CD patients carry the HLA-DQ2 protein, the rest carries HLA-DQ8 protein. The prevalence of the HLA-DQ2 and HLA-DQ8 in the general population is 25-40% compared to 1% prevalence of CD showing that the presence of these genotypes is necessary but not sufficient for the onset of the disease [2]. The answer lies in other genetic and environmental risk factors. Few studies have found that infant feeding practices, i.e., breastfeeding and age at gluten introduction, may influence the onset of CD [3456]. In this study, we study the influence of breastfeeding and timing of gluten introduction on the age at diagnosis of CD.
It was a hospital based observational prospective study done at the Department of Pediatrics, Swai Man Singh Medical College, Jaipur, from October 2011 to September 2012. Swai Man Singh Medical College and Attached Hospital Ethics Committee approved the study.
Patients diagnosed with CD (new and follow up cases) attending the out patient department or admitted in the hospital meeting the inclusion criteria: 1) diagnosed with symptomatic CD as per modified European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria (2012) [1]; 2) aged between 6 months to 6 years of age; and 3) giving informed written consent, were included into the study. Detailed history collected with the help of pre-design questionnaire with special emphasis on duration of breastfeeding and age of gluten introduction (from primary caretaker). Those who failed to answer the questionnaire and failed to meet the inclusion criteria excluded from the study. Patients examined clinically and routine investigations done. Data collected with the help of pre-designed proforma. During the study period of 1 year, 230 patients enrolled but 198 cases included in the study as per inclusion and exclusion criteria.
Exclusive breastfeeding is defined as “an infant's consumption of human milk with no supplementation of any type (no water, no juice, no nonhuman milk, and no foods) except for vitamins, minerals, and medications” [7].
The duration of the period for which infants exclusively or partially breastfed.
The age of the infant at dietary gluten introduction to diet for the first time, i.e., the first month postpartum during which time flour from wheat, rye, barley introduce.
The age of the cases when the diagnosis of CD done as per modified ESPGHAN [1] criteria.
Patients divided into two groups: 1) that were breastfed and 2) that were not breastfed at all. Breastfed children further assessed for exclusive breastfeeding and breastfeeding status during gluten introduction: 1) breast feeding discontinued before the month of gluten introduction; 2) breast feeding continued in the month of gluten introduction; or 3) breast feeding continued beyond the month of gluten introduction. Patients also divided according to age at gluten introduction: 1) <6 months and 2) >6 months. These groups studied to estimate the risk of infant feeding practices on the disease onset in the early years of life. Multiple linear regression analysis applied using the stepwise backward elimination method to study the relationship between dependent and independent variables. An association of independent and dependent variables studied to identify possible auto-correlation, and multi-co-linearity. The models were proposed and tested for the risk stratification of CD. Mean age of diagnosis of CD compared with the help of unpaired t-test between the patient those were breastfed and not breast fed. PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA) was used for all statistical analysis.
Table 1 enumerates the descriptive characteristics. Out of 198 cases, 185 had been breastfed as infants and 121 were exclusively breastfed for first 6 months. The difference in boys and girls regarding breastfeeding was insignificant. There was no significant difference in clinical presentation among various groups. The most common symptoms were weight loss, abdominal distension and chronic diarrhea (Table 2). Mean±standard deviation (SD) duration of symptoms was 10.5±6.6 months. Out of 198 cases, 30.30% (n=60) were diagnosed in the first 2 years of life. Among cases, those were not breastfed (n=14), 64.29% (n=9) had onset of symptoms in first 24 months of age as compared to 26.36% in breast fed group (p<0.01). In breastfed group, mean±SD age at diagnosis (3.68±1.55 years) was higher compared with other group (2.70±1.65 years) (p<0.05). In breastfed children mean±SD age of gluten introduction was also (10.65±4.74 months) significantly higher as compared to other group (7.35±3.13 months) (p<0.001). Those who discontinued breastfeeding before the month of gluten introduction had the youngest age at diagnosis of the disease (p<0.05) and shorter duration of breastfeeding (p<0.01; Table 3).
The age at onset of CD was under one year in 40.42% of the cases, who had started gluten before 6 months of age as compared to only 12.58% of those who had started gluten after 6 months (p<0.001). Thus, cases having gluten containing food introduction to their diet before 6 months of age had early onset of disease (Table 4).
The result of multiple linear regression statistical analysis showed that only two independent variables, i.e., “Breastfeeding Status during the Gluten Introduction” and “Age at the Gluten Introduction” significantly positively influencing the age at diagnosis of CD (p=0.000) (Table 5 and Fig. 1). The proposed model for risk stratification was tested and found statistically significant (ANOVA, F=18.842, p<0.05). R square was 0.131, i.e., 13.1% of variance explained by the model. There was no significant auto-correlation between the independent variables (auto-correlation between the respondents using Durbin-Watson statistic test was 1.96). In addition, there was no multi-co-linearity among the independent variables (variance inflation factor found below 10.0 for each independent variable). The tolerance of the independent variables influencing the independent variable found to be below the tolerable limit.
CD develops because of interaction between a dietary trigger and a genetic makeup of the host, with the possible influence of other environmental cofactors. The prevalence of the disease is increasing, possibly related to environmental factors and to increasing awareness [8]. The true prevalence of the disease is difficult to estimate due to wide variable clinical presentation of the disease. The symptomatic disease is just the tip of iceberg with high rates of asymptomatic individuals. European studies have estimated pediatric CD prevalence at approximately 1% [910]. In the last recent years, several epidemiological studies have suggested that early infant feeding practices may play an important role in the subsequent development of CD in childhood [34]. The geographical differences in CD may relate to different dietary patterns. In India, CD has high prevalence in high wheat-consuming northern states [11]. The incidence of CD is increasing in India [12].
Our study showed that breastfed children had older age at the diagnosis of CD, but simultaneously the number of children that were not breastfed was small. In addition, the breastfed children had delayed gluten introduction as compared to non-breastfed group. The result of multiple linear regression showed that the breastfeeding status at the time of gluten introduction is positively influencing the age at diagnosis of CD i.e., continuing breast feeding during gluten introduction to an infant's diet and extending it beyond delays the age at diagnosis of CD. There was no difference in timing of gluten introduction among these groups. Radlovic et al. [3] concluded that longer breastfeeding significantly reduced the risk that CD would manifest in the first year of life (odds ratio, −0.655; 95% confidence interval, 0.481-0.891; p=0.007). Mean age at diagnosis was significantly higher in infants, who had been breastfed at the time of gluten introduction (p<0.029) [3]. Akobeng et al. [4] in a meta-analysis concluded that children being breastfed at the time of gluten introduction had a 52% reduction in risk of developing CD. Many other studies also showed that breastfeeding is protective for the CD [131415]. All these studies, like ours, showed that continuing breastfeeding at the time of gluten introduction delayed the onset of the CD in early crucial years of rapid growth and development. However, it is not clear that breastfeeding provides permanent protection or merely delays the disease.
Our study also showed another variable, i.e., the age at gluten introduction in the infant's diet is positively influencing the age at diagnosis of CD i.e., delayed gluten introduction was associated with a later onset of the disease. Early gluten introduction may be harmful, but it cannot be sure because this group has been breastfed for shorter duration and breastfeeding had discontinued at time of gluten introduction. In addition, the amount of gluten consumed not measured. Norris et al. [6] concluded that the timing of gluten introduction to the infant diet is associated with the appearance of CD in children. However, Radlovic et al. [3] concluded there was no significant difference in age at diagnosis between infants, who had started consuming gluten before the fourth month or later.
We found in our study that delayed introduction of gluten after 6 months along with breastfeeding during gluten introduction provide protection against the symptomatic CD. This protective effect of breast feeding occurs via various mechanisms. Breast milk significantly protects against a number of infections including gastroenteritis [16]. Infections of the gastrointestinal tract in early life could lead to increased permeability of the intestinal mucosa, allowing the passage of gluten into the lamina propria. Gut infections, also known to increase tissue transglutaminase expression and this could favor the generation of deamidated gluten peptides, triggering CD in susceptible individuals [17]. Further, human milk IgA antibodies may diminish the immune response to ingested gluten by mechanisms such as agglutination of the antigen to immune complexes on the mucosal surface so that uptake is prevented [17]. The immune modulating property of human milk may exert through its T-cell specific suppressive effect. Experiments on peripheral lymphocytes stimulated with phytohaemagglutinin, OKT3 and alloantigen suggest an inverse relation between breastfeeding and autoimmune disorders as CD [18]. In addition, human milk provides many bioactive factors, including antimicrobial and anti-inflammatory agents, enzymes, hormones, and growth factors, many of which are involved in gut maturation and development of the infant's innate and acquired immunity [1920].
The recent position paper by ESPGHAN [21] stated that the breastfeeding does not influence the risk of disease onset in childhood and breastfeeding at the time of gluten introduction is not protective against the disease. However, previous cohort and observational studies had shown positive influence and the recent randomized control trials do not address this issue directly. Also the earlier introduction of gluten was associated with increased risk of CD in the early years of life, but the cumulative incidence was same when compared with delayed introduction of gluten. Szajewska et al. [22] in systemic review and meta-analysis also found that exclusive or any breastfeeding, as well as breastfeeding at the time of gluten introduction, did not reduce the risk of developing CD during childhood. They concluded that the later dietary gluten introduction was associated with later development of celiac specific autoimmunity and CD during childhood [2122]. They also found that consuming higher amount of gluten at weaning and during the first 2 years of life might increase the risk of CD during childhood [21]. Our study showed that delayed introduction of gluten along with continuing breastfeeding was associated with delayed onset of CD. However, our study had certain limitations: 1) It was an observational study; 2) the sample size was small; 3) the amount of gluten consume was not measured; and 4) our study was subjected to recall bias. Therefore, the role of infant feeding practices in the expression of CD requires further investigation by large multi-center trials. In the light of current World Health Organization recommendations on early infant feeding [23], it is necessary to highlight the favorable role of breastfeeding at the time of introduction of new food antigens in the infant's diet, including gluten, in order to develop the oral tolerance and prevention of the disease.
This study showed that breastfed children had older age at the diagnosis of CD. The age at gluten introduction and the breastfeeding status at the time of gluten introduction to an infant's diet positively influenced the age at diagnosis of CD, i.e., delayed gluten introduction to an infant's diet while the infant still being breastfed delayed the age at diagnosis of CD and the more prolonged breastfeeding was associated with a later onset of CD. Further studies are required to ascertain the role of infant feeding practices in expression of CD.
Figures and Tables
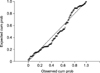 | Fig. 1Normal P-P plot of regression standardized residual dependent variable: age at diagnosis in months. Error chart showing that observed values of dependant variables is very closely related supporting the goodness of the fit of the model. |
Table 3
Relation of Breastfeeding Duration and Age at Diagnosis of CD with Breastfeeding Status during the Month of Gluten Introduction

References
1. Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012; 54:136–160.

2. Stanković B, Radlović N, Leković Z, Ristić D, Radlović V, Nikčević G, et al. HLA genotyping in pediatric celiac disease patients. Bosn J Basic Med Sci. 2014; 14:171–176.

3. Radlovic NP, Mladenovic MM, Lekovic ZM, Stojsic ZM, Radlovic VN. Influence of early feeding practices on celiac disease in infants. Croat Med J. 2010; 51:417–422.

4. Akobeng AK, Ramanan AV, Buchan I, Heller RF. Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child. 2006; 91:39–43.

5. Silano M, Agostoni C, Guandalini S. Effect of the timing of gluten introduction on the development of celiac disease. World J Gastroenterol. 2010; 16:1939–1942.

6. Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005; 293:2343–2351.

7. Tadele N, Habta F, Akmel D, Deges E. Knowledge, attitude and practice towards exclusive breastfeeding among lactating mothers in Mizan Aman town, Southwestern Ethiopia: descriptive cross-sectional study. Int Breastfeed J. 2016; 11:3.

8. Hernell O, Ivarsson A, Persson LA. Celiac disease: effect of early feeding on the incidence of the disease. Early Hum Dev. 2001; 65:Suppl. S153–S160.
9. Laass MW, Schmitz R, Uhlig HH, Zimmer KP, Thamm M, Koletzko S. The prevalence of celiac disease in children and adolescents in Germany. Dtsch Arztebl Int. 2015; 112:553–560.

10. Catassi C, Anderson RP, Hill ID, Koletzko S, Lionetti E, Mouane N, et al. World perspective on celiac disease. J Pediatr Gastroenterol Nutr. 2012; 55:494–499.

11. Gupta R, Reddy DN, Makharia GK, Sood A, Ramakrishna BS, Yachha SK, et al. Indian task force for celiac disease: current status. World J Gastroenterol. 2009; 15:6028–6033.

12. Sood A, Midha V, Sood N, Kaushal V, Puri H. Increasing incidence of celiac disease in India. Am J Gastroenterol. 2001; 96:2804–2805.

13. Greco L, Auricchio S, Mayer M, Grimaldi M. Case control study on nutritional risk factors in celiac disease. J Pediatr Gastroenterol Nutr. 1988; 7:395–399.

14. Peters U, Schneeweiss S, Trautwein EA, Erbersdobler HF. A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab. 2001; 45:135–142.

15. Fälth-Magnusson K, Franzén L, Jansson G, Laurin P, Stenhammar L. Infant feeding history shows distinct differences between Swedish celiac and reference children. Pediatr Allergy Immunol. 1996; 7:1–5.

16. Hanson LA, Korotkova M, Håversen L, Mattsby-Baltzer I, Hahn-Zoric M, Silfverdal SA, et al. Breast-feeding, a complex support system for the offspring. Pediatr Int. 2002; 44:347–352.

18. Mincheva-Nilsson L, Hammarström ML, Juto P, Hammarström S. Human milk contains proteins that stimulate and suppress T lymphocyte proliferation. Clin Exp Immunol. 1990; 79:463–469.

19. Rautava S, Walker WA. Academy of breastfeeding medicine founder's lecture 2008: breastfeeding--an extrauterine link between mother and child. Breastfeed Med. 2009; 4:3–10.

21. Szajewska H, Shamir R, Mearin L, Ribes-Koninckx C, Catassi C, Domellöf M, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016; 62:507–513.
22. Szajewska H, Shamir R, Chmielewska A, Pieścik-Lech M, Auricchio R, Ivarsson A, et al. Systematic review with meta-analysis: early infant feeding and celiac disease--update 2015. Aliment Pharmacol Ther. 2015; 41:1038–1054.

23. World Health Organization. Infant and young child feeding. Fact sheet. Updated January 2016. Available from: www.who.int/mediacentre/factsheets/fs342/en/www.who.int/mediacentre/factsheets/fs342/en/.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download