Abstract
Purpose
Foreign body (FB) ingestion is common in children, and button battery (BB) ingestion has been increasing in recent years. This study was to identify factors related to outcomes of FB ingestion, particularly BBs in the stomach. We evaluated whether the current recommendations are appropriate and aimed to suggest indications for endoscopic removal of BB in the stomach in young children.
Methods
We investigated patient age, shape, size, location of FBs, spontaneous passage time and resulting complications among 76 children. We observed types, size, location of BB and outcomes, and analyzed their associations with complications.
Results
Coins and BB were the two most common FBs. Their shapes and sizes were not associated with the spontaneous passage time. Size, spontaneous passage time, and age were also not associated with any specific complications. For BB ingestion, all 5 cases with lithium batteries (≥1.5 cm, 3 V) presented moderate to major complications in the esophagus and stomach without any symptoms, even when the batteries were in the stomach and beyond the duodenum, while no complications were noted in 7 cases with alkaline batteries (<1.5 cm, 1.5 V) (p=0.001). All endoscopies were conducted within 24 hours after ingestion.
Conclusion
The type and voltage of the battery should be considered when determining whether endoscopy is required to remove a BB in the stomach. For lithium battery ingestion in young children, urgent endoscopic removal might be important in order to prevent complications, even if the child is asymptomatic and the battery is smaller than 2 cm.
Foreign body (FB) ingestion is common in children. It has been reported that the ages of children ingesting FBs and the types of ingested objects vary greatly. Children often swallow coins, toy parts, jewelry, or batteries [1]. Most FB ingestions occur in children between 6 months and 3 years of age [2].
These patients may have no symptoms or could present with severe complications that require emergent medical attention [3]. Ingested FBs may pass spontaneously with time, or patients could experience complications, such as erosions, ulcers, and perforations [45]. We particularly focused on how some factors correlated with the patient's progress because of the several different possible outcomes.
Battery ingestion in particular is potentially life threatening; when it is lodged in the esophagus, this is a medical emergency that requires immediate removal. Despite prompt removal, severe complications and sequelae have been reported [67]. On the other hand, when it located in the stomach, it has been thought to be at low risk. 24-48 or more hours of observation are recommended. Recently, studies have reported complications caused by a button battery located in the stomach [8]; since then, revised guidelines have been suggested [910].
In this study, we identified a variety of factors associated with the ingestion of the FB, such as the type, size, and location of the objects ingested as well as the age of the children. We also investigated factors associated with complications especially concerning a button battery in the stomach. We aimed to evaluate if the current recommendations are appropriate and to suggest indications for endoscopic removal of a button battery in the stomach in young children.
We analyzed the medical records of 520 children under 18 years of age who visited Korea University Guro Hospital (Seoul, Korea) with complaints of FB ingestion from March 2012 through February 2015. We excluded subjects with no clear histories of ingestion, those who had the objects removed from their oral cavity or pharynx by otolaryngologists, children who ingested unidentified materials including dirt or powder, and subjects without complete follow-up medical records.
We assessed the patients' demographic characteristics, including sex and age; the shape, size, and location of the objects confirmed by endoscopy or radiography; whether upper endoscopy was conducted and the time period of any endoscopic procedure after FB ingestion; the time period in case of spontaneous passage; symptoms; and complications, including endoscopic findings when available.
In children who ingested a button battery, we also investigated the types, models, currents, and voltages of the batteries. Endoscopic findings were analyzed with relations to these factors to determine which are associated to complications (Fig. 1). Medical outcomes, including endoscopic findings, were defined modifying those of National Poison Data System of America as follow: (1) no complications: no signs or symptoms, (2) minor complication: minimal signs or symptoms that resolved rapidly, (3) moderate complication: more pronounced, prolonged, or multiple signs or symptoms, such as bloody stool or emesis, erosions, shallow ulcers or mucosal burns without sequelae, (4) major complication: significant residual disability, such as deep ulcers or mucosal burns with protracted healing or long-term sequelae, systemic effects, perforation, or fistula [10].
Data analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Categorical variables were assessed using the chi-square test and Fisher's exact test, and continuous variables were assessed using the Mann-Whitney U test and Kruskal-Wallis test. p<0.05 was considered to be significant for the statistical tests. The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of Korea University Guro Hospital (KUGH15123-001).
Of total 520 children, 76 children were enrolled. Of them, 44 were male (57.9%) and 32 were female (42.1%). The mean age was 3.13±3.07 years. Eleven children were younger than 12 months (14.5%), 18 (23.7%) were 12-23 months, and 11 (14.5%) were 24-35 months. Patients under 36 months of age made up 52.6% of the total patients (Fig. 2).
Seven patients (9.2%) showed symptoms of chest discomfort, abdominal pain, bloody stool, or bloodtinged saliva. Coins were the most common FB in 17 cases (22.4%): 9 cases (11.8%) of 100 Korean Republic Won (KRW) coin (diameter 2.4 cm), 4 cases (5.3%) of 10 KRW coin (2.29 cm, issued before December 2006). The second most common FB was a button battery in 12 cases (15.8%), followed by earrings in 5 cases (6.6%), a Go-stone in 4 cases (5.3%), and fish bones in 4 cases (5.3%) (Table 1). The mean size of the foreign bodies was 2.00±0.98 (range, 0.5-6 cm) except for the unidentified sizes of 4 fish bones. Objects sized ≥2 cm accounted for 50.0% (38 items).
Radiography and upper endoscopy were used to identify the location of the FB. Five objects (6.6%) were detected in the upper esophageal sphincter (UES), 3 (3.9%) in the mid- to lower esophageal sphincter (LES) and, most commonly, 30 (39.5%) in the stomach. Four objects (5.3%) were located in the duodenum, while 13 (17.1%) were distal to the duodenum, small bowel, and colon. Twenty-one objects (27.6%) were not observed with either radiography or upper endoscopy (Table 2).
Fifty-three of 76 children underwent upper endoscopy. The mean period of time from ingestion to the endoscopic procedure was 17.8±24.8 hours (range, 2-96 hours). Endoscopic removal succeeded in 31 cases; 1 underwent surgery due to perforation of the duodenum with a sharp object (a steel wire). In the remaining children, the FBs already moved past the duodenum, and spontaneously passed in the stool. The mean passage time was 36.9±29.7 hours (range, 10-124 hours).
The sharp pointed objects in 3 children took 26.7±15.3 hours (range, 10-40 hours) for spontaneous passage, while non-sharp objects took 40.0±32.9 hours (range, 11-124 hours) (p=0.573). Children with objects shorter than 2 cm took 39.1±32.0 hours (range, 10-124 hours) for spontaneous passage, and children with objects ≥2 cm took 25.0±7.1 hours (range, 20-30 hours) (p=0.553).
Nine of 76 (11.8%) children developed ulcers, erosions, and erythema on the endoscopy. Children with mucosal complications were 2.33±2.35 years of age, while those without complications were 3.14±3.29 years old (p=0.615). Of 18 children who swallowed objects <2 cm, 3 (16.7%) had mucosal complications. Out of the 29 children who ingested objects ≥2 cm, 6 (20.7%) showed mucosal complications (p=1.000). There was no statistical correlation between the spontaneous passage time and mucosal complications (p=1.000).
Of the 12 children who ingested button batteries, 8 were male (66.7%) and 4 were female (33.3%). The median age was 17 months (interquartile range [IQR], 12-31 months). Ten children were younger than 36 months, and 2 were ≥36 months. All were younger than 5 years, and none showed any symptoms after the ingestion.
The types of ingested batteries were alkaline manganese dioxide chemistry LR57 (diameter, 0.95 cm; 1.5 V, 40 mAh) in 2 children, LR44 (diameter, 1.16 cm; 1.5 V, 140 mAh) in 5 children, lithium manganese dioxide chemistry CR1616 and CR1620 (diameter, 1.6 cm; 3 V, 50-68 mAh) in 3 children, and CR2032 (diameter, 2.0 cm; 3 V, 210 mAh) in 2 children.
A button battery was detected in the UES in 1 child, in the stomach in 5, and distal to the duodenum in 6. Nine of 12 children underwent endoscopy. All 9 endoscopies were performed within 24 hours after ingestion. Upper endoscopy successfully removed the batteries with retrieval nets in 5 cases (1 was in the UES, and 4 were in the stomach). Seven objects passed spontaneously in the stool.
Complications were present in 5 (41.7%) of the total 12 children who ingested batteries. In one case, the button battery was located in the UES and was removed within 2 hours, but caused mucosal burns and deep ulcers with bleeding and exudate (Fig. 3). These sequelae remained when the patient was followed up (major complication). Complications were detected in 4 cases in the stomach, including 1 with a major complication and 3 with moderate complications (Fig. 4). Of them, in 1 case, the battery was lodged in the small bowel immediately before the upper endoscopy, but ulcers and erosions were identified in the stomach (moderate complication). The median age of 5 children with complications was 13 months (IQR, 11-35 months), while that of the 7 children without complications was 18 months (IQR, 14-31 months) (p=0.570).
All 7 children who ingested button batteries smaller than 1.5 cm did not show any symptoms or signs of complications, while all 5 children who swallowed batteries larger than 1.5 cm showed moderate (n=3) to major (n=2) complications. The 7 batteries that had no effect were alkaline batteries (LR57 and LR44), and all 5 that produced complications were lithium batteries (CR1616, CR1620, and CR2032). There was a significant statistical difference between the type of battery ingested (p=0.001) (Table 3). All of the 3 patients who did not go through endoscopy had no signs or symptoms and thus they were categorized as no complications according to National Poison Data System of America. Even if one case in esophagus and three cases without endoscopy are excluded, the analysis had statistically significant results as well (p=0.001).
The endoscopy time interval after ingestion was a median of 5.0 hours (IQR, 3-14 hours) in children with complications and a median of 4.5 hours (IQR, 3-11 hours) in those without complications who underwent endoscopy (p=0.806).
Whether upper endoscopy is performed in order to remove a FB depends on the time since the ingestion; the location, size, and shape of the FB; and the patient's age, symptoms, or complications. The guidelines have been revised by various committees through the years. In 2007, guidelines described by Seo recommended immediate endoscopic removal particularly for button batteries and sharp objects lodged in the esophagus [11]. If sharp or pointed objects, long objects (>4-5 cm for infants and young children, >6-10 cm for older children), or large and wide objects (>2 cm in diameter for infants and young children, >2.5 cm in diameter for older children) are located in the stomach, endoscopic removal is also recommended. In the case of a button battery ≥2 cm or that has remained for over 24-48 hours in the stomach, multiple magnets, or gastric retention of any objects for more than 3-4 weeks, endoscopic removal is recommended.
Recently, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) Endoscopy Committee revised the recommendations for the timing of endoscopic intervention [9]. If the FB lodged in the esophagus is a button battery, it should be removed within 2 hours. If the patient does not show any symptoms, 24 hours are allowed to observe them. In case long objects are ingested, they should be eliminated within 24 hours regardless of the presence of symptoms.
The frequency of button battery ingestion has been increasing due to widespread use of such batteries as power supplies in electronic devices [10]. It has been frequently reported that severe complications, including aortoesophageal fistula and vocal cord paralysis, are present in cases of a button battery in the esophagus [12131415]. Patients with a button battery in their esophagus should be managed with immediate endoscopic removal [91617].
However, most button batteries with a diameter <2 cm in the stomach pass spontaneously with no complications. If a battery with a diameter ≥2 cm stays in the pylorus or duodenum for a long time, it is recommended that it be removed out of concern for the increasing risk of perforation [11].
Recently, complications caused by a button battery located in the stomach seem to be increasing. In a case of a 3-month-old infant, the battery was firmly lodged in the antrum with its surface almost exposed on the serosa and was surrounded by necrotic debris located near the pylorus. After endoscopic removal failed, laparotomy and gastrotomy were conducted to remove the battery [8].
Litovitz et al. [10] reported worse outcomes for large diameter lithium batteries (≥2 cm) and in children who are younger than 4 years of age. They did not separate button batteries in esophagus and stomach. They suggested that endoscopic removal should be considered even if symptoms appear minor. If a child younger than 6 years is asymptomatic and the ingested battery is 1.5 cm in diameter or more, they recommended obtaining an X-ray within 4 days after ingestion. On the other hand, if the patient is older than 6 years or if the ingested battery is smaller than 1.5 cm, it can be managed at home and later confirmed that the battery has passed by inspecting the stool.
The NASPGHAN Endoscopy Committee suggested in 2015 that if children younger than 5 years of age have a button battery ≥2 cm in their stomach, an assessment for esophageal injury and endoscopic removal should be considered if possible within 24-48 hours. If the patient is 5 years or older or the battery diameter is <2 cm, outpatient observation may be an option to consider. For those children 5 years of age or older who have ingested a battery ≥2 cm in diameter, a repeat radiography in 48 hours is recommended. Clinicians should also consider repeating an X-ray if patients 5 years of age or younger have swallowed a battery <2 cm and fail to pass it in their stool within 10-14 days after ingestion. Endoscopic removal is required if gastrointestinal symptoms develop or the battery does not pass the stomach by the time the X-ray is repeated [9].
In our study, all cases associated with batteries >1.5 cm experienced complications (major complication, 2 cases; moderate complication, 3 cases). None of them showed any symptoms. We have a policy to remove button batteries in the stomach if the child's fasting time permits, especially when the size and type of the battery is not defined. We observed that in terms of endoscopic removal time, less than 2 hours should have elapsed since the button battery ingestion to remove it from the esophagus and 8 hours to remove it from the stomach, with the exception of 1 case where a button battery was removed after 20 hours. In our study, the button battery was properly removed, but complications were present. One child in particular who ingested a 1.6-cm lithium battery also presented with a major complication, even though the battery was removed within 5 hours. The two opposite rims of the battery were lodged in the antrum near the pylorus. Its surface was almost exposed on the serosa and was surrounded by necrotic debris and markedly thickened and inflamed peripheral mucosa. According to the recent recommendations of NASPGHAN [9] or Litcovitz et al. [10], we should have only conducted outpatient observation for 24 hours in 3 cases with complications related to a 1.6-cm battery, which could have led to more severe complications. Therefore, it might be reasonable to set 1.5 cm rather than 2 cm as the minimum size requirement for endoscopy in young children. Nelson Textbook of Pediatrics [18], the latest edition recommends that batteries larger than 1.5 cm are not likely to pass spontaneously and should be removed endoscopically in children younger than 6 years of age. In our study, complications were shown though button batteries were removed within 24 hours after ingestion. This is not the matter of spontaneous passage, so more urgent removal is needed to avoid complications by lithium batteries.
As all the children in this study were younger than 5 years, and most of them were younger than 3 years except for 2 children, we could not find a correlation between age and the complication rate.
There were 5 types of button batteries ingested by patients in our study. The battery diameters were 0.95 cm (LR57), 1.16 cm (LR44),1.6 cm (CR1616, CR1620), and 2.0 cm (CR2032). The product names complied with the required categorization by the International Electrotechnical Commission; "LR" represents the alkaline manganese dioxide chemistry, and "CR" represents the lithium manganese dioxide chemistry [19]. Lithium batteries are larger than alkaline batteries in general; however, the sizes of commercially available lithium batteries vary from 0.95 cm to 2.45 cm. The sizes of alkaline batteries range from 0.68 cm to 1.16 cm. The voltage of alkaline batteries ranged from 1.5 V to 1.55 V, while lithium batteries had a voltage of 3 V. According to Korea Consumer Agency, lithium battery smaller than 1.5 cm in diameter is rarely distributed in Korea.
In our study, the only batteries (1.6 cm and 2 cm) that produced complications were lithium batteries, while the other batteries (0.95 cm and 1.16 cm) that had no complications were alkaline batteries. Though the study sample was small, it can be considered that complications are related to higher voltages and specific types of batteries (lithium) rather than merely the size of the ingested battery. Since the percentage of lithium battery ingestion has increased from 1.3% in 1993 to 24% in 2008 [10], we suggest that battery types and voltages should be considered when making the decision to proceed with endoscopy.
Conventionally, the mechanisms of battery-induced injury have been explained: a leakage of the alkaline electrolyte, the generation of electrical current that hydrolyzes tissue fluids and produces hydroxide when it comes in contact with physiologic solutions, and physical pressure necrosis on adjacent tissues [20]. No severe complications were reported such as renal toxicity or hepatotoxicity even when heavy metal was systemically absorbed [2122].
New batteries also show a greater risk of injury than spent batteries. The higher incidence of severe injuries from lithium batteries, even those smaller than 2 cm in diameter, could indicate that the injury is related to the higher voltage rather than leakage of the alkaline electrolyte or the electrical current.
In summary, this study identified the types, sizes, and locations of FBs in children and analyzed their associated complications and spontaneous passage time. After FB passed through stomach, shapes and sizes were not associated with the spontaneous passage time and size, spontaneous passage time, and age were also not associated with any specific complications.
To our knowledge, this is the first cross-sectional study that showed mucosal complications in stomach caused by lithium batteries sized <2 cm. As the use of lithium batteries with higher voltages is increasing, factors such as the types and voltages of the battery should be considered when determining whether urgent endoscopy is required to remove a battery lodged in the stomach. In cases of young children with a lithium battery in the stomach, urgent removal could be important in order to prevent complications, even if the child is asymptomatic, even within 24 hours. Further study in multicenter is needed to revise the guidelines for removal of button battery, especially for the proper time of endoscopy.
Figures and Tables
Fig. 2
Age distribution (mean age, 3.13±3.07 years) of the 76 patients (44 boys [57.3%] and 32 girls [42.7%]) with foreign body ingestion.

Fig. 3
Endoscopic fingings of children with lithiuim battery (2.0 cm, 3 V) lodged in the upper esophageal sphincter (UES). The lithium battery was removed from the UES within two hours after ingestion. (A) Mucosal burns and two linear ulcerations with dirty exudate and bleeding were noted in the UES. (B) After five days, a linear deep ulceration with basal whitish exudate and peripheral mucosal edema was remained. A shallow linear ulceration was noted in the opposite side of the esophagus.
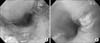
Fig. 4
Endoscopic fingings of children with lithiuim battery (1.6 cm, 3 V) lodged in the stomach. The lithium battery was removed from the stomach within five hours. (A) Linear ulcerations with blood clots and diffuse mucosal edema were noted in the body. (B) Ulcerations with dirty exudates and diffuse mucosal edema were noted in the antrum. (C) After three days, there was sign of mucosal traction accompanying surrounding edema along the two linear ulceration bases in opposing directions in the antrum.

References
1. Arana A, Hauser B, Hachimi-Idrissi S, Vandenplas Y. Management of ingested foreign bodies in childhood and review of the literature. Eur J Pediatr. 2001; 160:468–472.

2. Webb WA. Management of foreign bodies of the upper gastrointestinal tract. Gastroenterology. 1988; 94:204–216.

3. Kirkham EN, Kane M, Paul SP. Foreign body ingestion in children. Community Pract. 2015; 88:45–48.
4. Lee JH, Nam SH, Lee JH, Lee HJ, Choe YH. Spontaneous passage of gastrointestinal foreign bodies in children. Korean J Pediatr Gastroenterol Nutr. 2007; 10:157–165.

5. Litovitz T, Schmitz BF. Ingestion of cylindrical and button batteries: an analysis of 2382 cases. Pediatrics. 1992; 89:747–757.

7. Maves MD, Carithers JS, Birck HG. Esophageal burns secondary to disc battery ingestion. Ann Otol Rhinol Laryngol. 1984; 93:364–369.

8. Honda S, Shinkai M, Usui Y, Hirata Y, Kitagawa N, Take H, et al. Severe gastric damage caused by button battery ingestion in a 3-month-old infant. J Pediatr Surg. 2010; 45:e23–e26.

9. Kramer RE, Lerner DG, Lin T, Manfredi M, Shah M, Stephen TC, et al. Management of ingested foreign bodies in children: a clinical report of the NASPGHAN Endoscopy Committee. J Pediatr Gastroenterol Nutr. 2015; 60:562–574.
10. Litovitz T, Whitaker N, Clark L, White NC, Marsolek M. Emerging battery-ingestion hazard: clinical implications. Pediatrics. 2010; 125:1168–1177.

11. Seo JK. Endoscopic management of gastrointestinal foreign bodies in children: a clinical practice guideline. Korean J Pediatr Gastroenterol Nutr. 2007; 10:Suppl 1. 64–69.
12. Hamilton JM, Schraff SA, Notrica DM. Severe injuries from coin cell battery ingestions: 2 case reports. J Pediatr Surg. 2009; 44:644–647.

13. Mortensen A, Hansen NF, Schiødt OM. Fatal aortoesophageal fistula caused by button battery ingestion in a 1-year-old child. Am J Emerg Med. 2010; 28:984. e5–984. e6..

14. Virgilis D, Weinberger JM, Fisher D, Goldberg S, Picard E, Kerem E. Vocal cord paralysis secondary to impacted esophageal foreign bodies in young children. Pediatrics. 2001; 107:E101.

15. Soerdjbalie-Maikoe V, van Rijn RR. A case of fatal coin battery ingestion in a 2-year-old child. Forensic Sci Int. 2010; 198:e19–e22.

16. Sung SH, Jeon SW, Son HS, Kim SK, Jung MK, Cho CM, et al. Factors predictive of risk for complications in patients with oesophageal foreign bodies. Dig Liver Dis. 2011; 43:632–635.

17. Hung CW, Hung SC, Lee CJ, Lee WH, Wu KH. Risk factors for complications after a foreign body is retained in the esophagus. J Emerg Med. 2012; 43:423–427.

18. Judith RK, Chris AL. Foreign bodies and bezoars. In : Kliegman R, Stanton B, St Geme J, Schor NF, editors. Nelson textbook of pediatrics. 20th ed. Elsevier Saunder;2015. p. 1814–1816.
19. Galligan C, Morose G. An investigation of alternatives to miniature batteries containing mercury [Internet]. Lowell: Maine Department of Environmental Protection;2003. cited 2015 Aug 27. Available from: http://www.sustainableproduction.org/downloads/MaineDEPButtonBatteryReportFinal12-17-04.pdf.
20. Gryboski J. Traumatic injury. In : Walker WA, Durie PR, editors. Pediatric gastrointestinal disease. 3rd ed. Hamilton (ON): BC Decker;2000. p. 351–377.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download