Abstract
Purpose
To analyze the associations among the degrees of nonalcoholic fatty liver disease (NAFLD) by ultrasonography and metabolic syndrome, degrees of obesity in children, and degrees of parental obesity.
Methods
A total of 198 children with obesity who visited a pediatric obesity clinic were prospectively enrolled in this study. The severity of NAFLD based on ultrasonography was classified into no, mild, moderate, or severe NAFLD group. The degree of obesity based on the percentage over standard weight for height per sex was classified into mild, moderate, or severe.
Results
Of 132 patients evaluated for the degree of NAFLD and metabolic syndrome, the p-value of correlation between the two factors was 0.009. Therefore, metabolic syndrome might significantly affect the degree of NAFLD. Of 158 patients evaluated for the degree of NAFLD and the degree of obesity, the p-value of correlation between the two factors was 0.122. Of 154 patients evaluated for the degree of obesity and father's obesity, the p-value was 0.076. Of 159 patients evaluated for the degree of obesity and mother's obesity, the p-value was 0.000, indicating that mother's obesity could significantly affect the degree of obesity in children. Of 142 patients evaluated for the degree of obesity and metabolic syndrome, the p-value was 0.288.
Obesity is newly emerged as a very important health problem in children. The prevalence of obesity was 12.8% in school aged children (including elementary, middle and high school) of Korea in 2014 [1]. Obesity can be defined as body mass index (BMI, kg/m2) more than 95 percentile per sex and age or weight for height exceeding more than 120% of the standard weight for height per sex in children [2].
Nonalcoholic fatty liver disease (NAFLD) is one of the major complications in obese children and obese adults [3]. NAFLD can progress into liver cirrhosis and hepatocellular carcinoma [45]. NAFLD may be closely associated with metabolic syndrome [6]. However, few report has studied the severity of NAFLD associated with metabolic syndrome [6] and the degree of obesity. Furthermore, there is no report about the association between metabolic syndrome and the severity of NAFLD based on ultrasonography. Although BMI is the gold standard for diagnosing obesity, it cannot figure out the degree of obesity in children [2]. The degree of obesity in this study was based on the percentage over standard weight for height per sex. It was classified into mild, moderate, or severe.
Parental obesity can be closely associated with obesity in children [7]. However, at what degree that parental obesity can affect the degree of obesity in children remains unclear. Therefore, the objective of this study was to analyze the associations among the degree of NAFLD by ultrasonography, metabolic syndrome, degree of obesity in children, and parental obesity (Fig. 1).
The study prospectively enrolled 198 obese children who visited the pediatric obesity clinic of Jeju National University Hospital from March 2010 to February 2015 with BMI over 95 percentile per sex and age or weight for height more than 120%. Clinical information, results of laboratory tests, and liver ultrasonography were collected. The questionnaire for the beginning age of obesity in children was obtained from their parents. However, the beginning age of the obesity was only based on the recall of parents because anthropometric parameters could not be collected at that period.
Major clinical information included anthropometric data, the degree of obesity, BMI, blood pressure, and parental obesity. Parental obesity was defined when BMI was more than 25 kg/m2. The degree of obesity was defined as the percentage over standard weight for height per sex using the following formula: [(measured weight–standard weight for height per sex)/standard weight for height per sex]×100. The standard weight for height per sex was defined as the weight of 50th percentile according to the patient's actual height per sex based on the weight for height graph per sex provided in the Korean Child Growth Chart 2007. Degree of obesity was further graded into mild obesity (20-30%), moderate obesity (30-50%), and severe obesity (more than 50%).
Laboratory tests included lipid battery, liver functions tests, and serum levels of glucose, HbA1c, and insulin. Blood samples were collected from patients who had fasted for at least 8 hours. To evaluate insulin resistance, homeostatic model assessment-insulin resistance (HOMA-IR) was calculated using the following formula: fasting serum insulin (µU/mL)×fasting plasma glucose (mmoL/L)/22.5. In patients who had elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, differential diagnostic work-up was done by checking viral markers of hepatotropic virus and other viruses, serum ceruloplasmin level, muscle enzyme, et cetera.
The severity of NAFLD was classified into no NAFLD, mild, moderate, or severe NAFLD based on liver ultrasonography [8]. One radiologist performed ultrasonography for all patients. Liver biopsy was not performed in all patients. Mild NAFLD was defined as slightly increased liver echogenicity compared to kidney/spleen with prominent portal wall and diaphragm. Moderate NAFLD was defined as medium increase of liver echogenicity compared to kidney/spleen with dimness of portal wall and diaphragm. Severe NAFLD was defined as high increase of liver echogenicity compared to kidney/spleen without showing portal wall and diaphragm due to elevation of echo attenuation.
Metabolic syndrome was diagnosed for patients who had at least 3 factors among the following 6 metabolic risk factors suggested by Lambert et al. [9]: 1) obesity (more than 95th percentile of BMI per sex and age); 2) blood pressure more than 90th percentile per sex and age; 3) fasting serum triglyceride more than 110 mg/dL; 4) fasting high density lipoprotein (HDL) cholesterol less than 40 mg/dL; 5) fasting plasma glucose more than 110 mg/dL; 6) fasting serum insulin more than 20 µU/mL.
The study was performed after obtaining the approval from Institutional Review Board (IRB no. JNUH 201410013001-HE005). Statistical analyses such as the frequency (%) of variables, mean and standard deviation (SD) for continuous variables, χ2 test, and analysis of variance (ANOVA) test were performed using PASW Statistics ver. 18,0 (IBM Co., Armonk, NY, USA). Statistical significance was considered when p-value was less than 0.05.
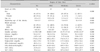
The mean age of the 198 obese children was 9.6±3.1 years-old (mean±SD). According to the degree of obesity, of the 198 obese children, 14.6% were mild, 52.5% were moderate, and 32.8% were severe (Table 1). Their beginning age of the obesity was 8.0±3.2 years old in the mild group, 6.4±3.2 years old in the moderate group, and 5.5±2.9 years old in the severe group (p=0.006, Table 1).
According to the degree of NAFLD based on ultrasonography, 159 obese children showed several significant findings among the 4 degrees of NAFLD (Table 2). Their age was 8.2±2.5 years old in the no NAFLD group, 10.2±3.0 years in the mild NAFLD group, 11.3±2.2 years in the moderate NAFLD group, and 12.9±1.8 years in the severe NAFLD group (p=0.000). Their age at the beginning of the obesity was 5.4±2.7 years old in the no NAFLD group, 6.5±3.3 years old in the mild NAFLD group, 7.4±2.9 years in the moderate NAFLD group, and 8.1 ±3.5 years old in the severe NAFLD group (p=0.002). Their fasting serum insulin level was 11.50±5.88 µU/mL in the no NAFLD group, 18.04±13.09 µU/mL in the mild NAFLD group, 23.37±17.14 µU/mL in the moderate NAFLD group, and 25.30±14.57 µU/mL in the severe NAFLD group (p=0.000). HOMA-IR value was 2.65±1.45 in the no NAFLD group, 4.23±3.20 in the mild NAFLD group, 5.57±4.38 in the moderate NAFLD group, and 5.96±3.72 in the severe NAFLD group (p=0.000). Their fasting serum triglyceride level was 84.1±39.1 mg/dL in the no NAFLD group, 91.3±38.5 mg/dL in the mild NAFLD group, 117.3±60.6 mg/dL in the moderate NAFLD group, 129.6±32.6 mg/dL in the severe NAFLD group (p=0.000). Their ALT levels showed the trend of higher level according to the severity of NAFLD.
The relationships between parental obesity and the degrees of obesity in children are shown in Fig. 2. Father's obesity was found in 9 of 23 (39.1%) children with mild degree of obesity, 46 of 81 (56.8%) children with moderate degree of obesity, and 33 of 49 (67.3%) children with severe degree of obesity (p=0.076). Mother's obesity was found in 3 of 24 (12.5%) children with mild degree of obesity, 25 of 86 (29.1%) children with moderate degree of obesity, and 27 of 49 (55.1%) children with severe degree of obesity (p=0.000). Both parental obesity was found in 9.1% of children with mild degree of obesity, 18.2% of children with moderate degree of obesity, and 40.0% of children with severe degree of obesity (p=0.004).
Regarding the relationship between metabolic syndrome and the degree of obesity or the degree of NAFLD, the comorbidity of metabolic syndrome according to the degree of obesity was not significant (p=0.288, Fig. 3A). The comorbidity of metabolic syndrome according to the degree of NAFLD degree was found in 14 of 68 (20.6%) children with no NAFLD, 12 of 29 (41.4%) children with mild NAFLD, 13 of 23 (56.5%) children with moderate NAFLD, and 6 of 12 (50.0%) children with severe NAFLD (p=0.005, Fig. 3B).
The comorbidity of NAFLD degree according to the degree of obesity was not significant (p=0.573, Fig. 4). The NAFLD degree did not have significant correlation with the degree of obesity either.
Although the definition of obesity has preferred BMI to the weight for height, BMI is not enough to give an easy explanation for the degree of obesity to obese children and their parents. Yoo et al. [10] have reported that percentage-weight-for-height (PWH) can reflect adiposity with higher sensitivity and specificity than the BMI. Although BMI can define body shape ranging from 'very skinny' to 'obese' [11], it has limitation in grading the degree of obesity. According to our study, the BMI of most children with obesity was more than 97 percentile per sex or age (Table 1). The grading of obesity by BMI was thought to be very difficult. Because obesity's definition by BMI should be based on its percentile instead of an absolute value, standardization of obesity degree in children is very difficult. PWH uses the standard weight for height in a child. Therefore, PWH can be used to standardize obesity degrees with absolute values. In this study, we graded the degree of obesity into mild (20% to 30%), moderate (30% to 50%), and severe (over 50%) based on the percentage over standard weight for height per sex. The grading for degree of obesity in this study was not correlated with clinical or laboratory parameters except for age at the beginning of the obesity (Table 1) and parental obesity (Fig. 2). Therefore, we could conclude that higher grading degree of obesity based on the percentage over standard weight for height does not reflect disease severity of child obesity.
This study revealed that the degree of NAFLD based on ultrasonography was significantly correlated with several clinical and laboratory parameters (Table 2). Among several imaging tests used to estimate the degree of NAFLD, ultrasonography is an inexpensive, noninvasive, and comfortable test to patient [1213]. It has been reported that ultrasonography has a sensitivity of 60% to 96% and a specificity of 84% to 100% for the detection of NAFLD when compared to liver biopsy in adults [1415]. Its test results could be dependent on the operator. Computed tomography has moderate sensitivity (82%) but high specificity (100%). However, it has radiation hazard [16]. Magnetic resonance image has a high sensitivity (100%) and moderate specificity (90.4%) [1718]. However, it is expensive and possibly uncomfortable to patient. In this study, only one expert radiologist performed ultrasonography for all patients.
When the age at the beginning of the obesity was older, the NAFLD degree was higher (p=0.000, Table 2). The severity of NAFLD was highly and significantly correlated with the level of serum insulin, HOMA-IR value, and the level of serum triglyceride (p=0.000). Although the p-value was significant (p=0.000), the severity of NAFLD was only moderately correlated with the levels of HDL-cholesterol, AST, and ALT due to reversed levels between moderate and severe NAFLD. Hyperinsulinemia has a major role in the formation of fatty liver [19]. Increase of insulin in the blood might be able to predict the degree of fatty liver, hepatic inflammation, and fibrosis [20]. Sundaram et al. [21] have reported that NAFLD in youth appears to be tightly correlated with components of the metabolic syndrome, especially visceral fat. Sundaram et al. [21] have insisted that visceral fat can predict fibrosis and liver fat. Park et al. [22] have shown that the risk of abdominal obesity in adults is proportionally increased to the degree of NAFLD. Therefore, central obesity is an important risk factor of NAFLD.
The degree of obesity in children was highly correlated with parental obesity, especially mother's obesity (p=0.000, Fig. 2B). When the grade of mother's obesity was greater, the degree of child obesity was higher, suggesting that mother's obesity can significantly affect the progress and the degree of child obesity. Therefore, to prevent or treat child obesity, we should also focus on mother's obesity. Whitaker et al. [7] have reported that parental obesity may more than double the risk of adult obesity among both obese and non-obese children. Many parents of obese children also might overlook their children's obesity [23]. Parents are the exclusive agent of change in the treatment of childhood obesity [24]. The degree of obesity based on PWH was not correlated with metabolic syndrome (Fig. 3A). Therefore, higher degree of obesity based on PWH does not mean higher rate of metabolic syndrome. However, Chang et al. [25] have reported that there is a trend of higher degree of obesity according to higher rate of metabolic syndrome. The degree of NAFLD was moderately correlated with metabolic syndrome, even though the reversed percentage value of metabolic syndrome was correlated with moderate and severe NAFLD (p=0.005, Fig. 3B). However, the degree of NAFLD was not correlated with the degree of obesity in children (Fig. 4). Patton et al. [6] have reported an association between metabolic syndrome and liver histology among children with NAFLD. They showed that the severity of NAFLD on liver histology was greater among children with metabolic syndrome vs. those without [6].
The limitation of the current study is the absence of normal weight group as control. Therefore, we could not compare each subgroup of obese children to normal weight children for the evaluation of associations among NAFLD degree, the degree of obesity, metabolic syndrome, and parental obesity. The second limitation of this study was that age at the beginning of the obesity was only based on recall of the parents instead of on anthropometric parameters at that period.
In conclusion, prevention and treatment for child obesity should also focus on parental obesity, especially mother's obesity. Severity grading of NAFLD by ultrasonography may be a good alternative method for liver biopsy. Additional and specialized management for metabolic syndrome may be necessary to prevent the progress of NAFLD.
Figures and Tables
 | Fig. 1Diagram of study design. The objective of this study was to analyze the associations among the degrees of nonalcoholic fatty liver disease (NAFLD) by ultrasonography and metabolic syndrome, degrees of obesity in children, and degrees of parental obesity. *The weight for height per sex at more than 120%. †Likelihood-ratio χ2 test. |
 | Fig. 2The relationship between the degree of obesity in children and parental obesity. Father's obesity percentage according to the degree of obesity in children (A) tended to be higher in the group with more severe degree of obesity. However, it was not significant (p=0.076, likelihood-ratio χ2 test). Mother's obesity percentage according to the degree of obesity in children (B) was higher in the group with more severe degree of obesity (p=0.000, likelihood-ratio χ2 test). Both parental obesity percentages (C) were higher in the group with more severe degree of obesity (p=0.004, likelihood-ratio χ2 test). |
 | Fig. 3The percentage of metabolic syndrome combined to the degree of obesity or the degree of nonalcoholic fatty liver disease (NAFLD). The percentage of metabolic syndrome combined to the degree of obesity (A) was not increased according to the increase in the degree of obesity (p=0.288, likelihood-ratio χ2 test). The percentage of metabolic syndrome combined to the degree of NAFLD (B) was higher in the group with more severe degree of NAFLD (p=0.005, likelihood-ratio χ2 test), even though the percentage between moderate and severe NAFLD was reversed. |
 | Fig. 4Relationship between the degree of obesity and the degree of nonalcoholic fatty liver disease (NAFLD). There was no significant change in the degree of NAFLD according to the degree of obesity (p=0.573, likelihood-ratio χ2 test). |
Table 1
Demographic and Clinical Characteristics of 198 Obese Children according to the Degree of Obesity

Values are presented as number (%), mean±standard deviation, or number only.
BMI: body mass index, HTN: hypertension, HOMA-IR: homeostatic model assessment-insulin resistance, HDL: high density lipoprotein, LDL: low density lipoprotein, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
References
1. Korean Education Ministry. The results for school health examination survey 2014 [Internet]. Sejong: Korean Education Ministry;2015. cited 2015 Feb 12. Available from: http://www.moe.go.kr/web/106888/ko/board/view.do?bbsId=339&boardSeq=58466.
2. Keane VA. Assessment of growth. In : Kliegman RM, Stanton BF, St Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Elsevier;2015.
3. Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005; 17:636–641.

4. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009; 58:1538–1544.

5. Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006; 43:413–427.
6. Patton HM, Yates K, Unalp-Arida A, Behling CA, Huang TT, Rosenthal P, et al. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010; 105:2093–2102.

7. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997; 337:869–873.

8. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:745–750.

9. Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int J Obes Relat Metab Disord. 2004; 28:833–841.

10. Yoo S, Lee SY, Kim KN, Sung E. Obesity in Korean pre-adolescent school children: comparison of various anthropometric measurements based on bioelectrical impedance analysis. Int J Obes (Lond). 2006; 30:1086–1090.

11. Ahn YM, Sohn M, Choi SH. Comparison in weight, height, degree of obesity and body mass index among different methods for body shape classification in school-age children. J Korean Acad Nurs. 2010; 40:775–784.

12. Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012; 54:700–713.

13. Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008; 7:212–220.

14. Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008; 48:442–448.

15. Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991; 43:26–31.

16. Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006; 239:105–112.

17. Radetti G, Kleon W, Stuefer J, Pittschieler K. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Paediatr. 2006; 95:833–837.

18. Pacifico L, Celestre M, Anania C, Paolantonio P, Chiesa C, Laghi A. MRI and ultrasound for hepatic fat quantification: relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatr. 2007; 96:542–547.

19. Mencin AA, Lavine JE. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2011; 14:151–157.

20. Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009; 50:1282–1293.

21. Sundaram SS, Zeitler P, Nadeau K. The metabolic syndrome and nonalcoholic fatty liver disease in children. Curr Opin Pediatr. 2009; 21:529–535.

22. Park SK, Ryoo JH, Choi JM, Seo MW, Park CM. The risk of abdominal obesity according to the degree of non-alcoholic fatty liver disease in Korean men. J Korean Med Sci. 2016; 31:410–416.

23. Etelson D, Brand DA, Patrick PA, Shirali A. Childhood obesity: do parents recognize this health risk? Obes Res. 2003; 11:1362–1368.

24. Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr. 1998; 67:1130–1135.

25. Chang JH, Kim DH, Kim HS, Choi IK, Cheong MY, Kim DK. Prevalence of metabolic syndrome in obese children. Korean J Pediatr. 2004; 47:1149–1156.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download