Abstract
Neonatal hemochromatosis (NH) is a severe neonatal liver injury that is confirmed by extra-hepatic iron accumulation. Although a recent study described treating NH with exchange transfusions and intravenous immunoglobulin, liver transplantation should be considered for patients with severe liver failure that does not respond to other medical treatment. Herein, we report the case of a two-month-old female infant who presented with persistent ascites and hyperbilirubinemia. Her laboratory findings demonstrated severe coagulopathy, high indirect and direct bilirubin levels, and high ferritin levels. Abdominal magnetic resonance imaging presented low signal intensity in the liver on T2-weighted images, suggesting iron deposition. The infant was diagnosed with NH as a result of the clinical findings and after congenital infection and metabolic diseases were excluded. The infant was successfully treated with a living-donor liver transplantation. Living related liver transplantation should be considered as a treatment option for NH in infants.
Neonatal hemochromatosis (NH) is defined as severe neonatal liver injury that is identified via extra-hepatic iron deposition [12]. While NH was initially thought to be an inborn error of iron metabolism, it is now accepted as an expressed phenotype of severe fetal liver injury. The most common cause of NH is gestational alloimmune liver disease (GALD), which originates from the placental passage of maternal Immunoglobulin G (IgG) antibodies that bind only to the fetal liver antigen [23]. The mechanisms involved include the activation of the terminal complement cascade by the classical pathway and the production of the membrane attack complex. NH hepatocytes that are immunostained with the monoclonal antibody to human C5b-9 complex (the terminal complement cascade neoantigen) present with more intense staining compared with non-NH hepatocytes [34]. Treatment with high-dose intravenous immunoglobulin (IVIG) during gestation in previously affected mothers has been found to significantly decrease the recurrence of NH, which also supports the alloimmune hypothesis [256].
Here, we report a case of NH in an infant who presented with severe liver failure. The infant was successfully treated with a living related liver transplantation from her mother.
A two-month-old female infant was transferred to Seoul National University Children's Hospital with abdominal distension and hyperbilirubinemia. She was born at 36 weeks 2 days of gestation via a normal spontaneous vaginal delivery and had a birth weight of 1.27 kg (<3rd percentile). Her Apgar scores were 6 at 1 minute and 9 at 5 minutes. Because she was small for her gestational age, she was admitted to the neonatal intensive care unit immediately after birth. Initially, hypoglycemia was noted and dextrose fluid was administered. Antenatal sonography findings presented no evidence of fetal hydrops or hepatomegaly. She had no familial medical history of gastrointestinal or hepatobiliary diseases. The patient's older brother was healthy and had experienced no medical problems. Her mother denied having any history of stillbirth or abortion.
The initial laboratory findings after birth showed the following increased values on the liver function test: aspartate aminotransferase (AST), 176 U/L; alanine aminotransferase (ALT), 106 U/L; total bilirubin, 1.76 mg/dL; and direct bilirubin, 1.2 mg/dL. Toxoplasmosis, syphilis, rubella, cytomegalovirus, herpes simplex virus and hepatitis B viral markers were examined under the suspicion of neonatal hepatitis, but all results were non-diagnostic. Abdominal sonography was performed to exclude biliary atresia, but there was no specific finding in the hepatobiliary tract. The patient was fed with preterm formula milk without feeding intolerance. No definitive sepsis event occurred during her hospitalization.
When the infant was 50 days old, a small amount of ascites was noted, and bilirubin levels had increased to a total serum bilirubin level of 10.89 mg/dL and a direct bilirubin level of 9.61 mg/dL. Coagulopathy was also detected. The amount of ascites increased thereafter, and ascites tapping was performed. The serum-ascites-albumin gradient level was elevated, and portal hypertension was suspected. By the age of 70 days, the infant was transferred to our hospital for further evaluation of persistent ascites suggesting portal hypertension and hyperbilirubinemia.
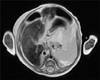
On the day of admission to our unit, the infant's height was 42 cm (<3rd percentile), her weight was 2.9 kg (<3rd percentile), and her head circumference was 33.5 cm (<3rd percentile). A physical examination indicated whole-body jaundice, a markedly distended abdomen with ascites and an umbilical hernia. The initial laboratory examination showed a white blood cell count of 13,600/µL, a hemoglobin level of 10.1 g/dL and a thrombocyte count of 93,000/µL. The liver function laboratory studies showed a total serum bilirubin level of 8.4 mg/dL, a direct bilirubin level of 6.4 mg/dL, an AST level of 225 U/L and an ALT level of 114 U/L. The albumin level was 2.4 g/dL, and the ammonia level was increased to 149 µg/dL. The prothrombin time was 31.2 seconds, and the international normalized ratio was 3.02. The serum ferritin level was elevated to 1,181.38 ng/mL (normal values are 14.0-647.2 ng/mL), and the transferrin saturation was 97.2% [7]. The serum alpha-fetoprotein level was in the high normal range (19,737.27 ng/mL; normal values are 40-19,953 ng/mL) [8]. Tyrosine was elevated in the plasma amino acid studies. However, no succinylacetone peak was found in the urine organic acid studies, which excluded the diagnosis of tyrosinemia. Abdominal magnetic resonance imaging (MRI) verified a diffuse heterogeneous low signal intensity in the liver on T2-weighted images, suggesting iron deposition (Fig. 1). An oral mucosal biopsy was performed to detect extra-hepatic siderosis, but adequate submucosal gland tissues could not be obtained. All of the studies performed to evaluate metabolic diseases presented non-diagnostic findings.
The infant received supportive medical treatment, including fat-soluble vitamins, ursodeoxycholic acid, fresh frozen plasma, lactulose, diuretics, and sodium benzoate. Her general condition and coagulopathy were aggravated, and her vital signs were unstable. She was transferred to the intensive care unit on day 18 of hospitalization for further management. Her mother began the donor evaluation process. Active gastrointestinal bleeding was observed on day 21 of the infant's hospitalization, and an emergency living donor liver transplantation was performed. The infant was 3 months old, and her weight was 3 kg on the day of transplantation. The graft was tailored by reducing its left lateral section. The pathological findings, which are presented in Fig. 2, revealed diffuse parenchymal iron deposition in the liver with iron staining; these findings were consistent with NH and distinct from mesenchymal iron deposition in cases of excessive iron supply [9]. The studies also revealed diffuse parenchymal collapse and occasional nodular regeneration, sinusoidal collagen accumulation and fibrosis, intracytoplasmic and intracanalicular cholestasis, and a decreased number of bile ducts with hematoxylin and eosin staining. The infant fully recovered from hepatic failure after liver transplantation. The infant was discharged one month after the operation. She exhibited normal renal function after transplantation. She received medical treatment for hyperkalemia, which occurred after the administration of tacrolimus. Following her discharge, the patient's neurological development was delayed, and she entered rehabilitation with outpatient clinic follow-up in the hospital's neurology department. Assessments by brain MRI and chromosomal study produced normal findings.
Intrauterine GALD is suspected to be a key factor in NH because most of the affected neonates are growth-restricted [210]. Fetal hydrops, hepatomegaly, and ascites may be observed during prenatal ultrasonography. The typical presentation of NH in infants includes hypoglycemia, coagulopathy and liver failure within a few hours or days after birth. However, some infants may not present severe symptoms [210]. Laboratory findings demonstrate severe coagulopathy, high ammonia levels, low albumin levels and relatively low serum aminotransferase levels for the liver injury grade. The indirect and direct bilirubin levels increase after birth, and alpha-fetoprotein and ferritin levels are elevated [23]. The patient in our study had intra-uterine growth restriction, but she presented relatively normal findings on prenatal sonography. Additionally, she had hypoglycemia, but hyperbilirubinemia was conclusively diagnosed after birth. Congenital infection, maternal autoimmune disease, metabolic disease and other disorders had to be excluded, which delayed the diagnosis. When the infant was 50 days old, she began to develop severe hepatic failure with ascites and was transferred to Seoul National University Children's Hospital.
Proven extra-hepatic iron deposition is necessary to confirm the diagnosis of NH and is generally confirmed via salivary gland biopsy [31112]. MRI may be another option for demonstrating extra-hepatic iron accumulation [313]. In the case of our patient, we could not obtain adequate tissues for the salivary gland biopsy, and the abdominal MRI only showed iron deposition in the liver; however, GALD could be diagnosed without evidence of extra-hepatic siderosis [14]. Additionally, the diagnostic sensitivity of oral mucosal biopsy and MRI is only approximately two-thirds [3]. Because the clinical findings could not rule out GALD, and because diagnoses such as congenital infection and metabolic diseases were excluded, we decided to perform liver transplantation under the tentative diagnosis of NH due to her clinical deterioration.
Other treatments using anti-oxidants and chelating agents have been performed to decrease iron overload. However, the survival rate for these treatments is lower than 20%. A recent study used treatments with exchange transfusions and IVIG that targeted the antibodies [2315]. The clinical outcomes improved with these treatments compared to previous treatments, and they decreased the need for liver transplantation [215]. These treatments would have been helpful for our patient if her symptoms had been mild.
NH is a common reason for liver transplantation in neonates [1617]. Liver transplantation should be considered for NH patients who do not respond to other medical treatments [1618]. The patient in our study underwent living donor liver transplantation to treat severe liver failure with coagulopathy. A recent study indicated that NH patients who underwent liver transplantation had lower patient and graft survival rates compared with patients in the general pediatric population who underwent liver transplantation. The main cause of mortality after transplantation was infection. The reasons for the graft failure were vascular thrombosis and primary non-function. The authors concluded that the graft failure was not caused by the disease itself but was mainly the result of technical problems caused by the small size of the NH patients [16].
The patient in our study underwent a living related liver transplantation from her mother. Some evidence indicates satisfactory outcomes of living related liver transplantation in children. In a previous study, biliary atresia patients who received living related liver transplants from their mothers had a lower incidence of graft failure than those who received liver transplants from their fathers. However, these results were not applied to liver transplant patients with other diagnoses [19]. Another study of biliary atresia patients reported that the father-to-daughter liver transplantation group had more acute cellular rejection than the mother-to-daughter group did. The authors agreed that the maternal antigens presumably influence graft tolerance in biliary atresia patients who undergo liver transplants [20]. Although these results were limited to biliary atresia patients, living related liver transplantation from the mother should be considered as an option for NH treatment. Maternal IgG antibodies, which only bind to fetal liver antigens, are considered to be the main reason for GALD, but those antibodies would not affect the transplanted maternal liver. In particular, mother-to-daughter liver transplantation would offer the benefit of a reduced chance of acute cellular rejection. The patient in our study was successfully treated with living related liver transplantation without notable complications 6 months after the treatment.
Figures and Tables
References
2. Lopriore E, Mearin ML, Oepkes D, Devlieger R, Whitington PF. Neonatal hemochromatosis: management, outcome, and prevention. Prenat Diagn. 2013; 33:1221–1225.

3. Whitington PF. Gestational alloimmune liver disease and neonatal hemochromatosis. Semin Liver Dis. 2012; 32:325–332.

4. Pan X, Kelly S, Melin-Aldana H, Malladi P, Whitington PF. Novel mechanism of fetal hepatocyte injury in congenital alloimmune hepatitis involves the terminal complement cascade. Hepatology. 2010; 51:2061–2068.

5. Whitington PF, Kelly S. Outcome of pregnancies at risk for neonatal hemochromatosis is improved by treatment with high-dose intravenous immunoglobulin. Pediatrics. 2008; 121:e1615–e1621.

6. Whitington PF, Hibbard JU. High-dose immunoglobulin during pregnancy for recurrent neonatal haemochromatosis. Lancet. 2004; 364:1690–1698.

7. Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, et al. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013; 59:1393–1405.

8. Soldin OP, Dahlin JR, Gresham EG, King J, Soldin SJ. IMMULITE 2000 age and sex-specific reference intervals for alpha fetoprotein, homocysteine, insulin, insulin-like growth factor-1, insulin-like growth factor binding protein-3, C-peptide, immunoglobulin E and intact parathyroid hormone. Clin Biochem. 2008; 41:937–942.

10. Whitington PF, Kelly S, Ekong UD. Neonatal hemochromatosis: fetal liver disease leading to liver failure in the fetus and newborn. Pediatr Transplant. 2005; 9:640–645.

11. Knisely AS, O'Shea PA, Stocks JF, Dimmick JE. Oropharyngeal and upper respiratory tract mucosal-gland siderosis in neonatal hemochromatosis: an approach to biopsy diagnosis. J Pediatr. 1988; 113:871–874.

12. Magliocca KR, Lewis EL, Bhattacharyya I, Cohen DM, Dixon LR. Labial salivary gland biopsy in the investigation of neonatal hemochromatosis. J Oral Maxillofac Surg. 2011; 69:2592–2594.

13. Hayes AM, Jaramillo D, Levy HL, Knisely AS. Neonatal hemochromatosis: diagnosis with MR imaging. AJR Am J Roentgenol. 1992; 159:623–625.

14. Debray FG, de Halleux V, Guidi O, Detrembleur N, Gaillez S, Rausin L, et al. Neonatal liver cirrhosis without iron overload caused by gestational alloimmune liver disease. Pediatrics. 2012; 129:e1076–e1079.

15. Rand EB, Karpen SJ, Kelly S, Mack CL, Malatack JJ, Sokol RJ, et al. Treatment of neonatal hemochromatosis with exchange transfusion and intravenous immunoglobulin. J Pediatr. 2009; 155:566–571.

16. Sheflin-Findling S, Annunziato RA, Chu J, Arvelakis A, Mahon D, Arnon R. Liver transplantation for neonatal hemochromatosis: analysis of the UNOS database. Pediatr Transplant. 2015; 19:164–169.

17. Sundaram SS, Alonso EM, Whitington PF. Liver transplantation in neonates. Liver Transpl. 2003; 9:783–788.

18. Rodrigues F, Kallas M, Nash R, Cheeseman P, D'Antiga L, Rela M, et al. Neonatal hemochromatosis--medical treatment vs. transplantation: the king's experience. Liver Transpl. 2005; 11:1417–1424.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download