Abstract
Pediatric mesenteric panniculitis is an extremely rare disease of unknown etiology characterized by chronic inflammation, fat necrosis, and fibrosis in the mesenteric adipose tissue. A previously healthy 13-year-old boy was admitted because of right upper abdominal pain. An abdominal computed tomography scan revealed increased attenuation and enhancement in the left upper abdominal omental fat and anterior peritoneal wall thickening. A laparoscopic biopsy showed mesenteric panniculitis with chronic inflammation, adiponecrosis, and septal fibrosis. Serological tests for autoimmune diseases, nested polymerase chain reaction for Mycobacterium tuberculosis, and special immunohistochemical stains for malignancy were all negative. Symptomatic improvement and improved abnormal findings were achieved after an 8-month treatment with prednisolone according to a follow-up abdominal computed tomography scan. Here, we report a case of pediatric mesenteric panniculitis treated with prednisolone.
Mesenteric panniculitis is an extremely rare disease of unknown etiology in children [123456], which is characterized by chronic inflammation, fat necrosis, and fibrosis in the mesenteric adipose tissue. Although the pathophysiology remains unclear, malignancy, trauma, tuberculosis, drugs, and antecedent surgery have been postulated as potential contributing factors in adults [789]. No consensus exists on the indications for treatment or an established regimen for managing mesenteric panniculitis, especially in child. In most of previous pediatric cases, the patients were observed without specific treatment or treated surgically [1234]. Here, we report a case of pediatric mesenteric panniculitis in a 13-year-old boy successfully treated with prednisolone.
A 13-year-old boy was admitted because of right upper abdominal pain accompanied by vomiting and diarrhea that developed 3 days before admission. No history of a specific disease, abdominal trauma, or surgery was revealed. Family history was unremarkable. His height was 156.5 cm (50-75 p), weight was 60.3 kg (90-97 p), and BMI was 24.8 kg/m2. Vital signs were normal except for low-grade fever (37.6℃), and tenderness over the right upper abdomen was noted without a palpable mass on a physical examination.
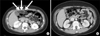
A blood test revealed the following: white blood cells (WBCs), 15,100/µL (neutrophils, 72.9%; lymphocytes, 18.2%); platelets, 454,000/µL; erythrocyte sedimentation rate, 29 mm/h; and C-reactive protein (CRP), 5.45 mg/dL. Liver function tests, amylase, lipase, ammonia, prothrombin time, partial thromboplastin time, antinuclear antibody, and antineutrophil cytoplasmic antibody were all negative. A chest radiograph showed no abnormalities. An abdominal computed tomography (CT) scan revealed increased attenuation and enhancement of the omental fat between the gall bladder and epigastric midline, as well as anterior peritoneal wall thickening and enhancement, suggesting omental fat inflammation or infarction (Fig. 1A). Laparoscopic biopsy was performed on hospital day (HD) 5, and the pathology showed mesenteric panniculitis with chronic inflammation, adiponecrosis, and moderate septal fibrosis (Fig. 2). Nested polymerase chain reaction for Mycobacterium tuberculosis and special immunohistochemical staining for β-catenin, calretinin, Ki-67, and P53 for malignancy were all negative.
Prednisolone was started (30 mg/d) on HD 6, and the abdominal pain subsided within 4 days. The blood tests showed improvement: WBCs, 7,320/µL (neutrophils, 55.6%; lymphocytes, 31.7%) and CRP, 1.65 mg/dL. He was discharged on prednisolone (30 mg/d) on HD 12, and the prednisolone was tapered to 10 mg/d over 1 month and continued at that dose for 7 months. An abdominal CT scan performed 8 months after discharge showed improvement from the previous abnormal findings without any significant sequelae (Fig. 1B). Follow-up blood testing was normal. The patient remained in good health after the 2 year clinical follow-up.
Mesenteric panniculitis is a rare disease and usually occurs in adults more than 50 years. Children are much rarely affected [123456], and as far as we know, this is the first mesenteric panniculitis in Korean child. In the present case, the exact cause was not found, even though we performed various tests, including serological testing for autoimmunity and pathological immunohistochemical staining.
An abdominal CT scan has been proposed to be the most effective diagnostic tool [10]. Two CT scan findings, such as a 'fat ring sign', reflecting preservation of fat around the mesenteric vessels and 'a tumoral pseudocapsule', representing a band of soft tissue that limits the uninvolved mesentery from the inflamed fat, are considered specific for mesenteric panniculitis [11]. Nevertheless, omental fat inflammation and infarction cannot be distinguished clearly by a CT scan, and a surgical biopsy and pathological analysis are usually necessary to make the diagnosis, as in the present case.
Three prominent pathological findings establish mesenteric panniculitis in terms of progression: chronic inflammation, fatty necrosis, and fibrosis. The clinical course of mesenteric panniculitis is generally favorable, and spontaneous remission has been reported [912]. Nevertheless it is associated with significant morbidity, and mortality under the diagnosis of retractile mesenteritis [1314]. Retractile mesenteritis which is the fibrotic end-stage of mesenteric panniculitis can result in mesenteric retraction, adhesion, and distortion of the intestinal loops [34815].
No consensus exists about the indications for treatment or an established regimen for managing mesenteric panniculitis. Treatment has been empiric and should be individualized. Immuno-suppressants, such as corticosteroids alone or with azathioprine usually more than 6 months may improve the disease course in adult [1617]. In most of previous pediatric cases, the patients were observed without specific treatment or treated surgically [1234]. One patient was misdiagnosed as appendicitis initially and diagnosed after surgery [6]. In one special case report, 6-year-old girl with small bowel obstruction was treated successfully with corticosteroid and methotrexate for more than 1 year [5]. In the present case, even though the diagnosis was not retractile mesenteritis, moderate fibrosis in the pathology and a high CRP level led us to start treatment, and 8 months of steroid treatment led to an excellent response in terms of blood and imaging tests. CRP levels may serve as a surrogate marker for the therapeutic response [18], and decrease in CRP was noted 4 days after starting prednisolone in the present case. Complete surgical resection is impossible in most cases, and is recommended only in cases of complications, such as intestinal obstruction or intestinal ischemia [4919].
The natural history of mesenteric panniculitis remains unclear because of its rarity and lack of adequate follow-up. However, benign, stable, or slowly progressive courses have been noted in most adult patients [20].
In this case, pediatric mesenteric panniculitis treated with prednisolone successfully. Mesenteric panniculitis is an extremely rare disease in children; however, it can be considered in children with acute or chronic abdominal pain with an abdominal mass.
Figures and Tables
References
1. Cakmak O, Tanyel FC, Cağlar M, Göğüş S. Mesenteric panniculitis mimicking acute abdomen in a 4-year-old child. Z Kinderchir. 1986; 41:313–314.

2. Hakgüder G, Akgür FM, Olguner M, Ozer E, Aktug T. A case of mesenteric panniculitis in a 4-year-old child. Pediatr Int. 2000; 42:577–578.

3. Davis CF, Guzzetta PC, Patterson K. Primary (retractile) mesenteritis in a child. J Pediatr Surg. 1992; 27:1544–1545.

4. Spark RB, Yakovac WC, Wagget J. Retractile sclerosing mesenteritis. Case report. Clin Pediatr (Phila). 1971; 10:119–122.
5. Viswanathan V, Murray KJ. Idiopathic sclerosing mesenteritis in paediatrics: Report of a successfully treated case and a review of literature. Pediatr Rheumatol Online J. 2010; 8:5.

6. Rumman N, Rumman G, Sharabati B, Zagha R, Disi N. Mesenteric panniculitis in a child misdiagnosed as appendicular mass: a case report and review of literature. Springerplus. 2014; 3:73.

7. Ferrari TC, Couto CM, Vilaça TS, Xavier MA, Faria LC. An unusual presentation of mesenteric panniculitis. Clinics (Sao Paulo). 2008; 63:843–844.

8. Ogden WW 2nd, Bradburn DM, Rives JD. Mesenteric panniculitis: review of 27 cases. Ann Surg. 1965; 161:864–875.
9. Durst AL, Freund H, Rosenmann E, Birnbaum D. Mesenteric panniculitis: review of the leterature and presentation of cases. Surgery. 1977; 81:203–211.
10. Horton KM, Lawler LP, Fishman EK. CT findings in sclerosing mesenteritis (panniculitis): spectrum of disease. Radiographics. 2003; 23:1561–1567.

11. Wat SY, Harish S, Winterbottom A, Choudhary AK, Freeman AH. The CT appearances of sclerosing mesenteritis and associated diseases. Clin Radiol. 2006; 61:652–658.

12. Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: a single entity? Am J Surg Pathol. 1997; 21:392–398.

14. Akram S, Pardi DS, Schaffner JA, Smyrk TC. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol. 2007; 5:589–596.

16. Tytgat GN, Roozendaal K, Winter W, Esseveld MR. Successful treatment of a patient with retractile mesenteritis with prednisone and azathioprine. Gastroenterology. 1980; 79:352–356.

17. Issa I, Baydoun H. Mesenteric panniculitis: various presentations and treatment regimens. World J Gastroenterol. 2009; 15:3827–3830.

18. Ginsburg PM, Ehrenpreis ED. A pilot study of thalidomide for patients with symptomatic mesenteric panniculitis. Aliment Pharmacol Ther. 2002; 16:2115–2122.

19. Parra-Davila E, McKenney MG, Sleeman D, Hartmann R, Rao RK, McKenney K, et al. Mesenteric panniculitis: case report and literature review. Am Surg. 1998; 64:768–771.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download