This article has been corrected. See "Correction: Characteristics of Pediatric Pancreatitis on Magnetic Resonance Cholangiopancreatography" in Volume 18 on page 216.
Abstract
Pediatric pancreatitis is not uncommon and results in considerable morbidity and mortality in the affected children. Unlike adults, pediatric pancreatitis is more frequently associated with underlying structural abnormalities, trauma, and drugs rather than an idiopathic etiology. Magnetic resonance cholangiopancreatography (MRCP) is a good imaging modality for evaluating pancreatitis and determining etiology without exposure to radiation. This article focuses on MRCP findings associated with various causes of pancreatitis in children, particularly structural abnormalities of the pancreaticobiliary system, as well as describing the feasibility, limitations, and solutions associated with pediatric MRCP.
Pancreatitis is more commonly encountered in children than ever before, although it remains unclear if the incidence itself has increased or just detection [123]. In contrast to adults, in whom the cause is often idiopathic, pancreatitis in children is usually caused by congenital structural abnormalities, trauma, drugs, and infection [345]. It has been reported that the structural abnormalities of the pancreaticobiliary system, including biliary stones and sludge, are the leading causes of recurrent pediatric pancreatitis [6]. Imaging studies, including ultrasound (US) and computed tomography (CT), are very important for diagnosing pancreatitis and identifying its causes. Generally, CT is superior to US for evaluating pancreatitis, regardless of patient age; however, CT uses ionizing radiation, which does potentially harm growing children. Magnetic resonance imaging (MRI) can be a good alternative for assessing children, and magnetic resonance cholangiopancreatography (MRCP) has become the standard imaging modality for evaluating pediatric patients with pancreatobiliary disease at many institutions. Recent studies have reported that MRCP demonstrates a fairly good correlation with endoscopic retrograde cholangiopancretography or direct cholangiography for delineating several structural abnormalities in the pancreaticobiliary system [78], which encourages the active use of MRCP for assessing in pediatric patients. This article provides a pictorial overview of the MRCP findings of pediatric pancreatitis according to the various causes, with a specific focus on the limitations and possible applications of pediatric MRCP.
Cross-sectional MRI is useful for assessing the extent of inflammation, peripancreatic fluid collection, and perfusion in the pancreatic parenchyma after administering paramagnetic contrast material. MRCP is indicated to evaluate structural abnormality of the pancreaticobiliary system in pediatric pancreatitis. The normal pancreas demonstrates high signal intensity relative to the liver on T1-weighted fat-suppressed images and low signal intensity relative to the liver parenchyma on T2-weighted images. The signal intensity of the inflamed pancreas is high on T2-weighted images, but remains normal or slightly low on T1weighted fat-suppressed images depending on the severity of pancreatic inflammation. Heavily T2-weighted image is sensitive to demonstrate the signal change of the pancreas parenchyma and peripancreatic fluid collection due to suppression of the fat tissue (Fig. 1A). Diffuse or focal dilatation and a discontinuous pancreatic duct can be seen on MRCP (Fig. 1B), but the small ductal anomalies might be obscured by inflammatory change in the acute stage of pancreatitis. Stones or sludge can be also identified in the pancreatic duct or common bile duct (CBD). In severe cases, there may be parenchymal necrosis and hemorrhage (so- called necrotizing or hemorrhagic pancreatitis) (Fig. 2).
Chronic pancreatitis is defined by irreversible architectural and functional changes in the pancreas due to long-standing pancreatic inflammation and injury [6]. It has been noted that chronic pediatric pancreatitis is mainly associated with obstructive and genetic causes [9]. MRCP can identify irregular ductal dilatation with or without stricture, calculi, and pseudocyst (Fig. 3 and 4). However, MRI has some limitations when identifying parenchymal calcifications and small pancreaticoliths or abnormalities in the small distal duct. Therefore, there have been attempts to combine MRI and unenhanced CT in order to diagnose chronic pancreatitis [10].
Pseudocyst can present as the complications of pancreatitis in both acute and chronic pancreatitis; they can also present either alone or in multiple. CT is the most sensitive imaging modality for detecting pancreatic pseudocysts, and MRCP is not usually solely used to identify pseudocysts, although it is good at depicting the solid portion within pseudocysts and delineating ductal communication (Fig. 3B).
Hereditary pancreatitis is an autosomal dominant disease that results from mutations in the cationic trypsinogen genes, including PRSS1 and SPINK1 [1112]. Recurrent episodes of acute exacerbation of pancreatitis result in chronic pancreatitis, especially in older children and adolescents. The MRCP findings of hereditary pancreatitis do not differ from those of acute and chronic pancreatitis, depending on the phase of the disease [13]. It is notable that PRSS1 and SPINK1 mutations are associated with pancreas divisum (PD), and these mutations in combination with PD can lead to chronic pancreatitis at an early age (Fig. 3 and 4) [14].
Autoimmune pancreatitis is histologically characterized by marked fibrosis and the infiltration of CD4-positive T-lymphocytes and immunoglobulin G4-positive plasma cells around the pancreatic duct [15]. Autoimmune pancreatitis is increasingly encountered in adults, but a few cases have been also reported in children and adolescents [16]. The representative characteristics of autoimmune pancreatitis on MR include the diffuse enlargement of the pancreas without focal lesions, multifocal pancreatic duct narrowing, and a peripancreatic hypodense or hypointense rim on contrast-enhanced CT or MRI (Fig. 5A and 5B). Improvement in the radiological findings after steroid therapy may be a diagnostic clue of autoimmune pancreatitis, as it usually indicates a good response to steroids.
Blunt trauma is one of the most common causes of pancreatic injury in children, resulting in >50% of pediatric cases [17]. The body of the pancreas is the most vulnerable site of blunt injury because it can be easily crushed against the vertebral column (Fig. 6A) [1718]. In an acute clinical setting, CT can be modality of choice regarding fast imaging time and accuracy for abdominal traumatic injury. MRI with MRCP is performed when the ductal injury is suspected although CT is the best modality for traumatic pancreatitis in acute clinical setting. Pseudocyst formation is common and has been reported in >50% of patients with traumatic pancreatitis. Approximately 60% of pancreatic pseudocysts are due to blunt trauma [17]. MRI and MRCP can reveal parenchymal changes, including pancreatic fracture, laceration, hemorrhage, pancreatic contusion, peripancreatic fluid collection, hematoma, pseudocyst formation, and other associated injuries in the adjacent abdominal organs (Fig. 6B) [18].
Drug-induced pancreatitis accounts for up to 12% of cases of acute pediatric pancreatitis [13], but only 0.1-2% of adult cases [19]. Drug-induced pancreatitis is particularly important in children with acute lymphoblastic leukemia (ALL) receiving L-asparaginase therapy, because acute pancreatitis can result. A recent study reported that the high mortality of ALL patients is due to coexisting acute pancreatitis [20]. Cytosine arabinoside treatment, hypercalcemia, and hypertriglyceridemia can also be associated with pancreatitis in ALL patients. Drug-induced pancreatitis also demonstrates a wide spectrum of disease manifestations, from subclinical pancreatitis to fatal hemorrhagic pancreatitis (Fig. 7).
Choledochal cyst (CDC) is a congenital anomaly that manifests as a dilated bile duct. Combination anomalous pancreaticobiliary ductal union (APBDU) and ductal stones can lead to bile reflux into the pancreatic duct which, in turn, predisposes CDC patients to the development of pancreatitis [2122]. MRCP is a valuable tool for preoperative evaluation because it can objectively determine the size, extent, and type of CDC, APBDU, and cholelithiasis, in addition to presence of pancreatitis (Fig. 8 and 9) [723]. The coexistence of CDC and PD is rarely reported (Fig. 10) [24]. On MRCP, CDC appears as a fluid-filled cystic dilation of the bile duct, which is best seen on T2-weighted imaging. CDC is categorized by the Todani classification system as follows [25]: type I (80-90%), cyst confined to the extrahepatic duct (Fig. 8 and 9); type II (3%), diverticulum of the extrahepatic duct; type III (5%), choledochocele, which is the dilatation of the intramural CBD that protrudes into the duodenum (Fig. 11); type IV (10%), multiple cystic dilatations in both the intra- and extrahepatic ducts (Fig. 10); type V, Caroli disease, manifests as multicystic dilatation of the intrahepatic bile duct and may be associated with renal cystic diseases.
In patients with APBDU, bile reflux can occur due to a dysfunctional sphincter of Oddi or because the common channel becomes obstructed by a stone, sludge, or protein plugs [26]. The maximum length of the normal common channel in children increases with age, and the maximum length of the common channel is reportedly 3 mm in infants and 5 mm in children between 3-15 years of age [2728]. A common channel >5 mm would be considered abnormal in children [7]. It may be difficult to identify APBDU on MRCP, particularly in young patients. A long common channel is represented as a single, tubular, high-signal intensity on MRCP after joining the CBD and pancreatic duct (Fig. 8, 9, 12). However, it may be difficult to identify APBDU on MRCP, particularly in young patients due to small caliber of the bile duct.
The majority cases of pediatric pancreatitis caused by bile duct stones are accompanied by structural anomalies in the pancreaticobiliary system, hemoglobinopathy, short bowel syndrome, and total parenteral nutrition [5]. On MRCP, CBD stones can be single or multiple and are typically observed as dark signal-filling defects with or without the dilatation of the bile duct or pancreatic duct. Stones demonstrate various signal intensities on T1-weighted imaging according to their composition (Fig. 8B), while demonstrating dark signal-filling defects on T2-weighted imaging and MRCP (Figs. 8, 9, 10, 12).
Although most patients with PD are asymptomatic, pancreatitis can develop when there is obstruction or stenosis in the accessory ampulla or the dorsal pancreatic duct that drains the major portion of the gland (Fig. 4 and 10) [2129]. In addition, PD can be associated with hereditary pancreatitis and PRSS1 and SPINK1 gene mutations (Fig. 3 and 4) [14]. However, it is questionable about the relationship between the high incidence of pancreatitis and the presence of PD [293031]. In patients with complete PD, the dorsal and ventral ducts are completely separated (Fig. 4), whereas incomplete divisum maintains a connection via the small branches (Fig. 12).
Respiratory motion artifacts are the most frequent cause of inadequate MRCP imaging, resulting in blurring and ghosting (Fig. 13) [283233]. This motion can result in inconsistent image quality because certain structures cannot be properly found in each sampled k-space, which can cause misdiagnosis because the normal pancreatic duct appears disconnected, stenotic, or dilated [34]. Respiratory motion artifacts can be minimized using the respiratory triggering technique. However, respiratory triggering lengthens the total scan time, especially in patients with irregular respiration due to respiratory misregistration. Moreover, averaging up to six excitations can reduce motion artifacts, although this also increases the scanning time.
Long echo time and echo train, small-caliber pancreaticobiliary duct, and respiratory motion artifacts contribute to low signal-to-noise ratio (SNR) on pediatric MRCP. Images with low SNR are dark and grainy, resulting in poor visualization of the pancreaticobiliary duct (Fig. 13). The general principles used to improve SNR on MRCP include averaging additional signals and using the proper field of view, greater number of phase-encoding steps, a highfield MR scanner, 3-dimensional (3D) MR sequencing, and selecting an appropriately sized radiofrequency coil [35].
Neonates and young infants have small bile ducts that measure approximately ≤1 mm in diameter, and the pancreatic duct is even smaller than the bile duct in normal patients. Such small structures produce fewer signals and tend to be more susceptible to respiratory motion artifacts. These problems could be solved using strategies that improve the SNR and reduce motion artifacts. In addition, secretin-stimulated MRCP can improve visualization of the pancreatic duct by triggering transient dilatation (Fig. 14) [10323637].
Fluid in the stomach, duodenum, small and large intestines, kidneys, and the cerebrospinal fluid in the spinal canal can be identified on MRCP (Fig. 12B and 15A). Pathological conditions such as a large number of ascites, periportal and peripancreatic fluid collections, and distended gallbladder also affect the visibility of the pancreatic duct. Such fluid can be reduced by fasting, choosing adequate slab thickness depending on the patient's body habitus (Fig. 15B), 3D sequencing with postprocessing (Fig. 15C), and administering negative oral contrast media such as ferumoxil oral suspension, ferric ammonium citrate, or pineapple juice [1032353839].
Various structural abnormalities of the pancreaticobiliary system contribute to the development of pancreatitis in children and they can be effectively evaluated by MRI and MRCP. In addition, MRI including MRCP is attractive imaging modality for use in children as it is free of radiation exposure and iodine contrast agent. Therefore, pediatric physicians and pediatric radiologist should be familiar with using MRCP to evaluate pancreatitis and its etiology in children.
Figures and Tables
 | Fig. 1A 4-year-old girl diagnosed with acute pancreatitis. (A, B) Axial single shot fast spin echo (half-Fourier acquisition single-shot turbo spin-echo) T2-weighted image and a single-shot radial acquisition with relaxation enhancement oblique axial image showing diffuse swelling that is worse in the tail of pancreas (asterisk), mild irregular dilatation of the pancreatic duct (open arrow), a dilated common bile duct (curved arrow), and peripancreatic fluid collection (arrow). |
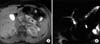 | Fig. 2A 14-year-old boy diagnosed with acute pancreatitis. (A) Fat-suppressed precontrast T1-weighted image showing high signal intensity in the body and tail of the pancreas (black asterisks), which is representative of parenchymal hemorrhage. (B) Magnetic resonance cholangiopancreatography image showing mild dilatation of the pancreatic duct that is abruptly cut-off at the tail of the pancreas (arrow). |
 | Fig. 3A 7-year-old boy with hereditary pancreatitis (SPINK1 mutation) presented with chronic pancreatitis. (A) Computed tomography image showing multiple parenchymal calcifications, irregular dilatation of the pancreatic duct, and parenchymal atrophy. (B) Maximum intensity projection 3-dimensional magnetic resonance cholangiopancreatography (MRCP) image showing diffuse dilatation of the pancreatic duct. The connection between the pseudocyst (asterisk) and pancreatic duct is shown (arrow). Note the minor duct crossing over the common bile duct (curved arrow) and pancreas divisum on the MRCP image. (C) Endoscopic retrograde cholangiopancretography confirming the connection between the pseudocyst and pancreatic duct. The contrasting agent filled the pseudocyst (open arrow). Note the irregular stricture of the pancreatic duct (arrow). |
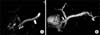 | Fig. 4A 4-year-old female with hereditary pancreatitis (SPINK1 mutation) and pancreas divisum. (A) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography (MRCP) image showing 3 pancreatoliths in the downstream minor pancreatic duct (arrowheads). Note the diffuse dilation of the pancreatic duct (arrow) (B) Volume-rendering 3-dimensional MRCP image clearly depicting the dilated minor pancreatic duct crossing over the distal common bile duct (arrow), which drains into the minor papilla (pancreas divisum). The irregularity of the upstream duct can also be seen (curved arrow). |
 | Fig. 5An 11-year-old boy diagnosed with autoimmune pancreatitis. (A) Fat-suppressed T2-weighted image showing diffuse pancreatic enlargement and increased signal intensity in the parenchyma (asterisk). Note the capsule-like low-signal rim surrounding the pancreas (curved arrow). (B) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography image showing the segmented absence of the pancreatic duct in the body and tail of the pancreas (between arrowheads) and the irregular dilatation of the upstream duct (arrow). (C) Follow-up magnetic resonance image obtained 20 days later shows less pancreas swelling after steroid therapy. |
 | Fig. 6A 4-year-old male with a history of blunt abdominal trauma. (A) Reformatted coronal contrast-enhanced computed tomography scan showing fracture at the pancreas neck (arrow) and peripancreatic fluid collection (arrowheads). (B) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography images obtained 17 days after injury, showing a pseudocyst (asterisk) connected to the collected fluid at the fracture site (curved arrow). |
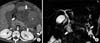 | Fig. 7An 11-year-old male diagnosed with acute lymphoblastic leukemia who was treated using L-asparaginase. (A) Contrast-enhanced computed tomography scan showing acute necrotic collections (arrows). The area of high attenuation (asterisk) may indicate hemorrhage. (B) Maximum intensity projection 3-dimensional magnetic resonance cholangiopancreatography image obtained 2 months later showing walled-off necrosis at the neck and tail of the pancreas (arrows). Dark signal intensities (curved arrow) may indicate hemosiderin deposits. |
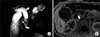 | Fig. 8A 3-year-old female diagnosed with acute pancreatitis caused by a type I choledochal cyst and associated with anomalous pancreaticobiliary ductal union and choledocholithiasis. (A) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography image showing fusiform dilatation of the extrahepatic bile duct (type I choledochal cyst, arrow), long common channel (curved arrow), and a stone in the common channel (arrowhead). Note the diffuse dilatation of the pancreatic duct (open arrow). (B) Axial T1-weighted image showing a stone within the common channel (arrow). |
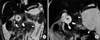 | Fig. 9A 1-year-old female with acute necrotizing pancreatitis caused by a type I choledochal cyst and anomalous pancreaticobiliary ductal union. (A) Coronal T2-weighted image showing slightly increased signal intensity in the pancreas (arrow), a large amount of ascites, and a choledochal cyst (asterisk). Note the acute necrotic collections (arrowheads) and stone in the long common channel (curved arrow). (B) Maximum intensity projection 3-dimensional magnetic resonance cholangiopancreatography image showing union between the pancreatic duct and bile duct (arrow), which makes a long, common channel that measures approximately 10 mm. Note the choledochal cyst (asterisk) and the large acute necrotic collections (arrowheads), as well as the mild dilatation of the pancreatic duct (curved arrow). |
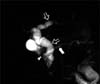 | Fig. 10A 3-year-old male with acute pancreatitis caused by a choledochal cyst and stone in the minor pancreatic duct. Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography image showing the diffuse dilatation of the intra- and extrahepatic bile duct (open arrows), which is indicative of type IV choledochal cyst. Note the prominent minor duct with irregular dilatation (arrow) and the filling defect at the opening to the minor papilla, which suggests a stone (arrowhead). Pancreas divisum was confirmed on endoscopic retrograde cholangiopancretography (not shown). |
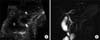 | Fig. 11A 7-year-old male with acute recurrent pancreatitis caused by a choledochocele cyst. (A) Ultrasonography revealing union between the common bile duct (arrow) and pancreatic duct (arrowhead) with bulbous dilatation within the ampulla of Vater. A choledochocele cyst is protruding into the second portion of the duodenum ("D"). (B) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography image showing pancreaticobiliary ductal union that protrudes into the duodenum (curved arrow). |
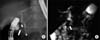 | Fig. 12A 3-year-old-female diagnosed with acute pancreatitis accompanied by a type I choledochal cyst, anomalous pancreaticobiliary ductal union, and atypical incomplete pancreas divisum. (A) Endoscopic retrograde cholangiopancretography (ERCP) demonstrating the fusion of the dorsal (short arrow) and ventral pancreatic duct (arrowhead) that drains into the minor papilla (long arrow). Note mild and fusiform dilatation of the common bile duct that is connected to the ventral duct. The filling defect within the common bile duct is bowel gas. Major papillae are not depicted on ERCP. (B) Single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography showing the long common channel draining into the major papilla (arrow). Note the stone in the common channel (arrowhead), and which was not seen on ERCP. |
 | Fig. 13Degraded image quality due to respiratory motion artifacts and poor signal-to-noise ratio. Note the blurring of the structures and the dark, grainy image. |
 | Fig. 14A 16-year-old boy diagnosed with chronic pancreatitis. (A) This single-shot radial acquisition with relaxation enhancement magnetic resonance cholangiopancreatography image fails to show the pancreatic duct. (B) The pancreatic duct was well visualized 4 minutes after the administration of secretin (arrow). |
 | Fig. 15Elimination of the obscuring structures. (A) Single-shot, 50-mm-thick radial acquisition with relaxation enhancement (RARE) image showing the obscured pancreaticobiliary system due to the presence of fluid in the stomach (arrow). (B, C) Single-shot, 20 mm-thick RARE image and maximum intensity projection 3-dimensional magnetic resonance cholangiopancreatography image showing the eliminated signal due to unnecessary fluids. |
ACKNOWLEDGEMENT
We wish to thanks to Dr. Hee Mang Yoon, Dr. Young Ah Cho, Dr. Jin Seong Lee, and Dr. Chong Hyun Yoon (Asan Medical Center, Seoul, Korea) for preparing the figures, and we are grateful to Dr. Yeoun Joo Lee (Pusan National University Yangsan Hospital, Yangsan, Korea) for her assistance in preparing the manuscript.
References
1. Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr. 2002; 140:622–624.

2. Nydegger A, Heine RG, Ranuh R, Gegati-Levy R, Crameri J, Oliver MR. Changing incidence of acute pancreatitis: 10-year experience at the Royal Children's Hospital, Melbourne. J Gastroenterol Hepatol. 2007; 22:1313–1316.

3. Werlin SL, Kugathasan S, Frautschy BC. Pancreatitis in children. J Pediatr Gastroenterol Nutr. 2003; 37:591–595.

4. Benifla M, Weizman Z. Acute pancreatitis in childhood: analysis of literature data. J Clin Gastroenterol. 2003; 37:169–172.
5. Choi BH, Lim YJ, Yoon CH, Kim EA, Park YS, Kim KM. Acute pancreatitis associated with biliary disease in children. J Gastroenterol Hepatol. 2003; 18:915–921.

6. Su WJ, Chen HL, Lai HS, Ni YH, Chang MH. Pancreaticobiliary anomalies is the leading cause of childhood recurrent pancreatitis. J Formos Med Assoc. 2007; 106:119–125.

7. Fitoz S, Erden A, Boruban S. Magnetic resonance cholangiopancreatography of biliary system abnormalities in children. Clin Imaging. 2007; 31:93–101.

8. Tipnis NA, Dua KS, Werlin SL. A retrospective assessment of magnetic resonance cholangiopancreatography in children. J Pediatr Gastroenterol Nutr. 2008; 46:59–64.

9. Clifton MS, Pelayo JC, Cortes RA, Grethel EJ, Wagner AJ, Lee H, et al. Surgical treatment of childhood recurrent pancreatitis. J Pediatr Surg. 2007; 42:1203–1207.

10. Matos C, Cappeliez O, Winant C, Coppens E, Devière J, Metens T. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002; 22:e2.

11. Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996; 14:141–145.

12. Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000; 25:213–216.

13. Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009; 29:1003–1026.

14. Lee YJ, Kim KM, Choi JH, Lee BH, Kim GH, Yoo HW. High incidence of PRSS1 and SPINK1 mutations in Korean children with acute recurrent and chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2011; 52:478–481.

15. Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010; 45:787–793.

16. Blejter J, Weller S, Pace R, Cusumano H, Giambini D. Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg. 2008; 43:1368–1372.

17. Leva E, Huscher C, Rode H, Fava G, Napolitano M, Maestri L, et al. Management of traumatic complete pancreatic fracture in a child: case report and review of literature. J Laparoendosc Adv Surg Tech A. 2008; 18:321–323.

18. Yang L, Zhang XM, Xu XX, Tang W, Xiao B, Zeng NL. MR imaging for blunt pancreatic injury. Eur J Radiol. 2010; 75:e97–e101.

19. Balani AR, Grendell JH. Drug-induced pancreatitis: incidence, management and prevention. Drug Saf. 2008; 31:823–837.
20. Treepongkaruna S, Thongpak N, Pakakasama S, Pienvichit P, Sirachainan N, Hongeng S. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol. 2009; 31:812–815.

21. Kuhn JP, Slovis TL, Haller JO, Caffey J. Caffey's pediatric diagnostic imaging. 10th ed. Philadelphia: Mosby;2004.
22. Okada A, Nakamura T, Higaki J, Okumura K, Kamata S, Oguchi Y. Congenital dilatation of the bile duct in 100 instances and its relationship with anomalous junction. Surg Gynecol Obstet. 1990; 171:291–298.
23. Suzuki M, Shimizu T, Kudo T, Suzuki R, Otsuka Y, Nagata S, et al. Usefulness of non-breath-hold one shot magnetic resonance cholangiopancreatography for the evaluation of choledochal cyst in children: 189*. J Pediatr Gastroenterol Nutr. 2005; 41:551–552.

24. Sonoda M, Sato M, Miyauchi Y, Yazumi S, Nakamura M. A rare case of choledochocele associated with pancreas divisum. Pediatr Surg Int. 2009; 25:991–994.

25. Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977; 134:263–269.
26. Mori K, Nagakawa T, Ohta T, Nakano T, Kadoya N, Kayahara M, et al. Acute pancreatitis associated with anomalous union of the pancreaticobiliary ductal system. J Clin Gastroenterol. 1991; 13:673–677.

27. Guelrud M, Morera C, Rodriguez M, Prados JG, Jaén D. Normal and anomalous pancreaticobiliary union in children and adolescents. Gastrointest Endosc. 1999; 50:189–193.

28. Philpott C, Rosenbaum J, Moon A, Bekhit E, Kumbla S. Paediatric MRCP: 10 year experience with 195 patients. Eur J Radiol. 2013; 82:699–706.

29. Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004; 60:419–425.

30. Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985; 89:951–958.

31. Hayakawa T, Kondo T, Shibata T, Sugimoto Y, Kitagawa M, Suzuki T, et al. Pancreas divisum. A predisposing factor to pancreatitis? Int J Pancreatol. 1989; 5:317–326.
32. Delaney L, Applegate KE, Karmazyn B, Akisik MF, Jennings SG. MR cholangiopancreatography in children: feasibility, safety, and initial experience. Pediatr Radiol. 2008; 38:64–75.

33. Chavhan GB, Almehdar A, Moineddin R, Gupta S, Babyn PS. Comparison of respiratory-triggered 3-D fast spin-echo and single-shot fast spin-echo radial slab MR cholangiopancreatography images in children. Pediatr Radiol. 2013; 43:1086–1092.

34. Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001; 21:23–37.

35. Chavhan GB, Babyn PS, Manson D, Vidarsson L. Pediatric MR cholangiopancreatography: principles, technique, and clinical applications. Radiographics. 2008; 28:1951–1962.

36. Dreiling DA, Messer J. The secretin story: a saga in clinical medicine and gastrointestinal physiology. Am J Gastroenterol. 1978; 70:455–479.
37. Geenen JE, Hogan WJ, Dodds WJ, Stewart ET, Arndorfer RC. Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology. 1980; 78:317–324.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download