Abstract
Heterotopic pancreas (HP) is defined as pancreatic tissue lacking anatomic and vascular continuity with the main body of the pancreas. Most are asymptomatic, but can cause ulcer, bleeding, intussusception, and mechanical obstruction. Herein, we presented one case of HP presented as duodenal tumor causing duodenal obstruction. A 7-year-old girl visited the emergency room for abdominal pain with vomiting for 24 hours. Computed tomography and upper gastrointestinal series revealed a polypoid mass with short stalk in the 2nd portion of duodenum. We attempted an endoscopic removal. However, the lumen was nearly obstructed by the mass and the stalk was too broad and hard to excise. The mass was surgically removed via duodenotomy. It was confirmed as a HP with ductal and acini components (type 2 by Heinrich classification). Postoperatively, the patient has been well without any complication and recurrence.
Heterotopic pancreas (HP) is defined as pancreatic tissue lacking anatomic and vascular continuity with the main body of the pancreas [1]. Almost all are asymptomatic and reportedly identified in 0.6% to 13% autopsies [234]. It could be found in the entire gastrointestinal tract and mostly in stomach, duodenum, and jejunum nearby pancreas [45]. The symptom varies from ulcer, bleeding, intussusception, and mechanical obstruction. We described a case of HP presented as duodenal tumor causing duodenal obstruction.
A 7-year-old girl visited the emergency room for abdominal pain with 10 times of vomiting within 24 hours. It was the first time she had. She felt a dull ache intermittently. She was healthy without any prior illness. Her height was 130.1 cm (97 percentile) and the weight was 29 kg (90-95 percentile). She was slightly dehydrated but not severely ill looking. On physical examination, her belly was very soft and there was no abdominal tenderness. The hemoglobin was 12.9 g/dL and other laboratory findings including amylase, lipase, and liver profile were within normal range. Simple abdomen revealed a 3 cm sized soft tissue density near the pylorus without small bowel obstruction (Fig. 1). We checked computed tomography and noticed a well demarcated mass near the ampulla of Vater in the 2nd portion of duodenum (Fig. 2). Ultrasonography and upper gastrointestinal series revealed a polypoid mass with short stalk in the 2nd portion of duodenum (Fig. 3 and 4). We attempted endoscopic examination and removal. However, the lumen was nearly obstructed by the mass and the stalk was too broad and hard to excise. We therefore decided on surgical resection. On laparotomy, we could palpate the mass after Kocher's maneuver. It was an approximately 3 cm well-demarcated mass broadly attached to duodenal wall. We opened the anti-mesenteric side of duodenum. The base of mass was attached to mesenteric side of duodenum just above the ampulla of Vater (Fig. 5). We completely removed the mass without injury of ampulla of Vater and pancreas parenchyma. The duodenotomy was closed transversely with a single-layer absorbable suture. The patient could take sips of water after 3 days of operation and recovered uneventfully. Pathologically, there were multifocal scattered nodular lesions, up to 0.3×0.2 cm in diameter in the submucosa and muscularis propria (hematoxylin and eosin [H&E] stain, ×40 or ×100). Microscopically nodular lesions were composed of variably dilated ductular mucinous and pancreatic exocrine acinar tissue (H&E, ×200). Immunohistochemistry of acini tissue showed positive reaction to CK19 (CK19, ×200). It was confirmed as HP, ductal and acini component (type 2 by Heinrich classification [5]) (Fig. 6). Postoperatively, the patient has been well without any discomfort and there was no abnormal finding in computed tomography during the 1 year follow-up period.
Duodenal obstruction is very rare in school aged children excluding congenital causes such as duodenal web or membrane. Rather, possible causes are foreign body ingestion, post-ulcer scar, trauma related duodenal hematoma and tumor. Intussusception of duodenum is very rare because the posterior wall of duodenum is mostly fixed to the retroperitoneum. Brunner's gland hyperplasia or duodenal tumor such as lipoma and polyp are reportedly possible causes of intussusception [67].
In this case, we found the duodenal obstruction was caused from HP. It included all histological components of normal pancreas except the main pancreatic anatomy and vascular continuity. The mechanism of HP is still unclear, but abnormal signaling pathways during the development of the pancreas or abnormal embryonic development of the pancreas are possible causes. If an evagination of the wall of duodenum is located on the bowel wall, it can form ectopic tissue anywhere in the gut [8]. HP has been classified into 3 types according to the histologic components (Heinrich classification). Type I is composed of complete structures consisting of ducts, acini, and islets of Langerhans cells. Type II is composed of ducts and acini. Type III is composed of ducts alone [5]. Histologic type is not related to the clinical symptoms and severity of disease [19]. Rather, the symptom from HP depends on the site, size, mucosal involvement, and pancreatic diseases of heterotopic pancreatic tissue itself [8]. Mucosal ulceration can lead to gastrointestinal bleeding, and vague symptoms such as abdominal pain, dyspepsia, and acid regurgitation [123456789]. Armstrong et al. [10] found that HP larger than 1.5 cm is more likely to be clinically symptomatic. Occasionally, HP causes gastric outlet obstruction or intussusception of the small bowel [39]. Duodenal obstruction as occurred in the current case was rarely reported than gastric outlet obstruction [39]. HP also can show islet cell proliferation causing severe hypoglycemia and even malignant change [8].
The diagnosis of HP before surgery is difficult. Even in stomach lesion, endoscopic finding and endoscopic ultrasonography revealed submucosal lesion that could not be confirmed by biopsy. Some are removed endoscopically with satisfactory results, but it can be considered in select cases depending on the size and location of the mass, especially for treating the benign lesions of HP [3]. The treatment of HP is controversial, because of most patients with HP are asymptomatic [391112]. It was reported that medical treatment can control the symptoms of HP [11]. However, asymptomatic patients of 4.2-6.4% of HP with Meckel's diverticulum needed surgical treatment [1314]. Liu et al. [8] suggested early surgical treatment is appropriate to confirm the diagnosis and to avoid serious complications regardless if asymptomatic. In summary, we agree that HP should be removed whenever it is symptomatic or it is incidentally found during laparotomy [12].
We reported the case of duodenal obstruction caused by HP and reviewed the relevant literature. Besides malignant duodenal obstruction, ulcer related complication and benign tumor such as lipoma, leiomyoma, and HP could be a cause of duodenal obstruction. Transduodenal tumor excision is a good surgical option for benign masses, as in our case. However, injury to the ampulla of Vater should be avoided.
Figures and Tables
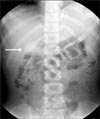 | Fig. 1Simple abdomen revealed a 3 cm sized soft tissue density near the pylorus without small bowel obstruction (arrow). |
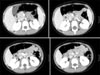 | Fig. 2Computed tomography revealed a well demarcated mass near the ampulla of Vater in the 2nd portion of duodenum. |
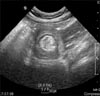 | Fig. 3Ultrasonography showed hyperechoic mass-like lesion with short stalk in the 2nd portion of duodenum. |
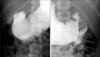 | Fig. 4Upper gastrointestinal series revealed ovoid-shaped filling defect in the 2nd portion of duodenum. |
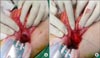 | Fig. 5After Kocher's maneuver, we identified the mass and exposed it after duodenotomy. We identified the mass (A) and exposed it after duodenotomy (B). |
 | Fig. 6Under the microscope, nodular lesions were composed of variably dilated ductular mucinous and pancreatic exocrine acinar tissue (H&E; A: ×40, B: ×200). Acini tissue showed positive reaction to CK19 immunohistochemistry (C: ×40, D: ×200). It was confirmed as heterotopic pancreas, ductal and acini component (type 2 by Heinrich classification). |
References
1. Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986; 151:697–700.
2. Tolentino LF, Lee H, Maung T, Stabile BE, Li K, French SW. Islet cell tumor arising from a heterotopic pancreas in the duodenal wall with ulceration. Exp Mol Pathol. 2004; 76:51–56.

3. Jiang LX, Xu J, Wang XW, Zhou FR, Gao W, Yu GH, et al. Gastric outlet obstruction caused by heterotopic pancreas: a case report and a quick review. World J Gastroenterol. 2008; 14:6757–6759.

4. De Castro Barbosa JJ, Dockerty MB, Waugh JM. Pancreatic heterotopia; review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet. 1946; 82:527–542.
5. Kinoshita H, Yamaguchi S, Shimizu A, Sakata Y, Arii K, Mori K, et al. Adenocarcinoma arising from heterotopic pancreas in the duodenum. Int Surg. 2012; 97:351–355.

6. Jennings BS, Doerr RJ. Duodenal lipoma causing intussusception. Surgery. 1989; 105:560–563.
7. Lempke RE. Intussusception of the duodenum: report of a case due to Brunners gland hyperplasia. Ann Surg. 1959; 150:160–166.
8. Liu YM, Shen HP, Li X, Gong JP. Heterotopic pancreas: a clinical analysis of nine patients and review of literature. Am Surg. 2012; 78:E141–E143.

9. Ormarsson OT, Gudmundsdottir I, Mårvik R. Diagnosis and treatment of gastric heterotopic pancreas. World J Surg. 2006; 30:1682–1689.

10. Armstrong CP, King PM, Dixon JM, Macleod IB. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981; 68:384–387.

11. Yoon HC, Koh YS, Kim JC, Cho CK, Kim HJ, Lee WS, et al. Ectopic pancreas presenting as a duodenal obstructing mass. J Korean Surg Soc. 2005; 68:65–68.
12. Ogata H, Oshio T, Ishibashi H, Takano S, Yagi M. Heterotopic pancreas in children: review of the literature and report of 12 cases. Pediatr Surg Int. 2008; 24:271–275.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download