Abstract
Purpose
Primary sclerosing cholangitis (PSC) is a rare condition that can be associated with inflammatory bowel disease (IBD). The aim of this study was to evaluate PSC and its association with IBD in children.
Methods
We retrospectively enrolled 13 pediatric patients (<18 years) with PSC treated at Asan Medical Center between June 1989 and December 2013. Clinical findings and long-term outcomes were investigated. During the same period, the incidence of PSC among IBD patients was evaluated among 600 Crohn disease (CD) and 210 ulcerative colitis (UC) patients.
Results
All 13 study patients diagnosed with PSC also presented with IBD. Eleven boys and two girls with a median age of 15.0 years old (9.0-17.8 years) were included. The cumulative incidence of PSC for UC was 5.7% (12 of 210) and 0.2% for CD (1 of 600), respectively. PSC occurred during follow-up for IBD for five patients (38.5%) whereas, IBD developed during follow-up for PSC for two patients (15.4%), and was diagnosed during the initial work-up for PSC for 6 patients (46.2%). For the 77.3 month median follow-up period, 9/13 patients (69.2%), neither the clinical symptoms nor blood test results worsened. Two cases (15.4%) developed liver cirrhosis and underwent liver transplantation. Among 13 PSC patients with IBD, two (15.4%) developed colorectal cancer, and no one developed cholangiocarcinoma.
Primary sclerosing cholangitis (PSC) is a chronic hepatobiliary disease with findings that include a beaded pattern of dilatation, stricture, and occlusion in the intrahepatic and extrahepatic bile ducts on cholangiography [123]. The clinical progress of patients with PSC is variable. In some cases, after undergoing biliary liver cirrhosis due to the fibrosis and stricture of the intrahepatic and extrahepatic bile ducts, as well as portal hypertension, and hepatic failure, patients with PSC may require liver transplantation [145]. In addition, PSC is closely related to the onset of cholangiocarcinoma or colon cancer, thereby requiring regular follow-up of the patient's clinical progress [678].
Cases of comorbid PSC and inflammatory bowel disease (IBD) and the comorbidity levels in adults are variable, as reported in various countries. In a study conducted with adult American PSC patients in 1989, IBD comorbidity was 71% [7]. In a cohort study conducted with 604 PSC patients between 1970 and 1998 in Sweden, IBD comorbidity was 80% [6]. According to a report in 2011 in Japan, PSC and IBD comorbidity in adults was 68.9%, indicating that comorbidity is also high in Asia [9]. Cases of PSC and IBD comorbidity reported in children are rare compared to those reported in adults. Kaplan et al. [10] reported the PSC prevalence rate among adults as 1.11 cases per 100,000 person-years, but the PSC prevalence rate among children was only 0.23 cases per 100,000 person-years. However, in a 2001 study that examined PSC and IBD comorbidity in children, the comorbidity of IBD among patients <18 years of age was high, at 83% [11].
Among the studies reported in South Korea to date-although cases of children diagnosed with PSC have been reported [121314]-no study has yet looked at the process of diagnosing children with PSC, at IBD comorbidity, or at treatment and clinical progress. The present study analyzes the characteristics of pediatric patients diagnosed with PSC at the Asan Medical Center, whether or not the condition was associated with IBD. The study analyzed clinical and histological characteristics, clinical progress and treatment, and liver cirrhosis and colon cancer incidence rates during the follow-up period.
This study was a retrospective case series of 13 patients with PSC under 18 years of age diagnosed at the Asan Medical Center (Seoul, Korea) between June 1989 and December 2013. The cumulative incidence of PSC among IBD patients was calculated according to the number of IBD patients who had been diagnosed with ulcerative colitis (UC; n=210) or Crohn disease (CD; n=600) in the same period. The study was approved by the institutional review board of the Asan Medical Center.
Patients were diagnosed with PSC according to a laboratory test, liver histology, endoscopic retrograde cholangiopancreatography (ERCP) findings, and magnetic resonance cholangiopancreatography (MRCP) findings. PSC was suspected in those cases where the alkaline phosphatase (ALP) or γ-glutamyl transferase (GGT) increased to more than 50% above the normal values for their age groups [15]. Liver histology was defined by the presence/absence of bile duct damage, onion-skinned periductal fibrosis, inflammation, portal edema or fibrosis, ductopenia, ductular proliferation, or cholestasis [1]. With regard to imaging characteristics, patients were diagnosed with PSC when the ERCP or MRCP indicated multifocal strictures, focal dilatation, or beading of the biliary tree [16]. Patients whose biochemical and histological tests indicated PSC but whose imaging tests showed normal biliary tree findings were diagnosed with small duct PSC [17]. Patients with sclerosing cholangitis due to secondary causes such as surgery, trauma, cancer, or infection were excluded from the study. A complete blood count, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP, GGT, total and direct bilirubin, albumin, prothrombin and activated partial thromboplastin time, IgG, antinuclear antibody, anti-smooth muscle antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were conducted at the time of diagnosis through blood tests. Special blood tests and urinalyses were carried out to exclude other immunological diseases such as acute viral hepatitis, and Wilson's disease. In cases where patients were diagnosed at other hospitals and transferred to our center, the results of biochemical, histological, and imaging tests at other hospitals were reviewed again at the study center. In cases where PSC was suspected in patients who were already being followed due to UC or CD, an ERCP or MRCP was carried out.
Once a patient was diagnosed with PSC, a colonoscopy was conducted for IBD screening both in cases where the patient complained of gastrointestinal symptoms and in cases where there were no symptoms. A definitive IBD diagnosis was made according to the patient's medical history, physical examination, and laboratory, radiologic, endoscopic, and histologic criteria described elsewhere [18].
During the median follow-up period of 77.3 months, the patients visited the outpatient clinic periodically to enable their AST, ALT, ALP, GGT, and bilirubin values to be checked. All patients diagnosed with PSC took ursodeoxycholic acid (UDCA) continuously. Patients diagnosed with IBD took sulfasalazine or 5-aminosalicylates (5-ASA), and steroids, immunosuppressants, or infliximab were administered when any of the patients showed acute exacerbation.
Among the 13 patients that participated in the present study, 11 were male and 2 were female. Their median age when diagnosed was 15.0 years (range, 9.0-17.8 years). All 13 study patients diagnosed with PSC also presented with IBD. Twelve (92.3%) had UC, and one (7.7%) had CD as the first presentation, and PSC was diagnosed during follow-up for five patients (38.5%); whereas, for two patients (15.4%), PSC was the first presentation and IBD was diagnosed later during follow-up. For six patients (46.2%), PSC presented first, and IBD was diagnosed subsequently at the time of their PSC diagnosis during their initial work-up for PSC (Table 1).
All 13 patients suffered from IBD. Twelve patients associated with UC and one patient associated with CD. Therefore, the cumulative incidence rates of comorbid PSC were 5.7% for UC (12 of 210) and 0.2% for CD (1 of 600), respectively.
Common clinical symptoms at the time of diagnosis were jaundice, itching, and abdominal pain in four cases (30.8%), as indicated in Table 2. In the laboratory results, 53.8% (n=7) of the study patients showed increased AST and ALT levels, with values of 144.9±215.8 U/L (normal range, 15-40 U/L) and 88.8±112.5 U/L (normal range, 5-45 U/L), respectively. Their total and direct bilirubin levels were 5.1±7.7 mg/dL and 4.1±7.7 mg/dL, respectively. GGT was measured in 12 patients, and all patients showed elevated GGT values over a range of 138.0±105.3 U/L (normal range, 5-25 U/L). When compared to normal values by age group (the normal range in a 10- to 15-year-old male is 116-483 U/L and in an age-matched female is 93-386 U/L; in a 16- to 19-year-old male is 58-237 U/L and in an age-matched female is 45-116 U/L), two children (15.4%) showed increased ALP with values in the range of 287.5±219.7 U/L. The ratio of patients with total bilirubin in the normal range was 38.5% (n=5) in tests conducted when the ERCP or MRCP results indicated PSC.
Nine patients (69.2%) underwent an ERCP, three (23.1%) underwent both an ERCP and a MRCP, and one (7.7%) underwent a MRCP alone. All 13 patients showed multifocal strictures, focal dilatation, or beading of the biliary tree on cholangiography, as expected in a PSC diagnosis (Fig. 1).
Among biliary involvements identified through cholangiography, cases with abnormal findings in the intrahepatic duct were the most frequent, affecting eight patients (61.5%). Involvement of both intrahepatic and extrahepatic ducts was evident in five cases (38.5%). Results indicating a PSC diagnosis were identified in all 13 patients undergoing an ERCP and a MRCP. In the present study, therefore, no patient was diagnosed with small duct PSC (Table 1).
In the present study series, two patients (15.4%) underwent a liver biopsy. According to the biopsy results, both patients showed onion-skinned periductal fibrosis and inflammation, portal fibrosis, ductopenia, ductular proliferation, and cholestasis conditions-criteria matching a PSC diagnosis (Fig. 2).
During 77.3 months of median follow-up (range, 25.5-285.2 months), all patients began to take UDCA and 5-ASA (n=13). Some also took systemic steroids (n=6) depending on their IBD activity. During the most recent follow-up period, a remission was maintained in 11 patients (84.6%).
Among the 13 study patients, 2 (15.4%) underwent liver transplantation. The first patient was simultaneously diagnosed with the intrahepatic type of PSC and UC at 15 years of age. The diseases progressed into liver cirrhosis at 17 years of age, and the patient underwent a liver transplantation four years later at 19 years of age. The other patient was diagnosed with PSC at 17 years of age while being followed-up after an UC diagnosis. This patient underwent a liver transplantation four years later at 21 years of age. We have continued to follow this patient, so far without any particular problem. As for UC progress, both are taking 5-ASA and have maintained their remission states. No one developed cholangiocarcinoma in our study.
Colon cancer developed in two male patients (15.4%). One was diagnosed with UC, PSC, and colon cancer at the ages of 10, 26, and 30 years old. Following a proctocolectomy, the patient is disease-free during 3 years of follow-up. The other patient was diagnosed with UC, PSC, and colon cancer at the ages of 15, 16 and 28 years old with mortality.
During the long-term follow-up of the other patients, nine patients neither underwent liver transplantation nor were diagnosed with colon cancer. One was first diagnosed with both the intrahepatic and extrahepatic types of PSC, and then with UC at 14 years of age. This patient died during the ambulatory follow-up period for unknown reasons. We continue to follow the remaining eight patients (61.5%) as outpatients while they are being continuously treated.
In conclusion, a Kaplan Meier survival analysis was carried out, based on the clinical progress of the patients with both PSC and IBD (n=13), who were followed up for long periods of time. On the basis of follow-up for mean 12.1 years (median, 11.4 years), the incidence ratio of major medical complications such as liver transplantation, cancer, and death was 0.38 (5/13), and the mean time of occurrence was 14.0 years.
The present study introduces the largest number of cases of children with PSC-IBD in a domestic single-center study. Major outcomes in our current study series included analysis of both the natural course of the disease and IBD comorbidity, and the incidence of major medical complications such as liver transplantation, colon cancer and death through long-term follow-up of patients under 18 years of age when diagnosed with PSC. Because all the cases with complications involved patients with PSC-UC, our present findings demonstrated that the relevant facts were recognized and the need for periodic follow-up was clarified.
With regard to other studies conducted with PSC-IBD patients, when 47 patients with PSC were analyzed through a retrospective medical record review, the mean age at the time of diagnosis was 11 years, the IBD comorbidity was 59%, the rate of pancolitis findings through colonoscopy was 24%, and the rate of rectal sparing findings was 24% [11]. In another retrospective medical record review study conducted with 52 PSC patients, IBD comorbidity was high, at 84%. Of the relevant patients, 89% were diagnosed with UC and 11% were diagnosed with CD, indicating that PSC-UC was predominant. During follow-up, 80% of colonoscopy findings indicated pancolitis; whereas, 26.7% showed rectal sparing [11]. A large cohort study conducted with 1,649 IBD patients in the early 2000s reported PSC incidence rates five years after IBD diagnosis as 0.5% (95% confidence interval [CI] 0.2-13%) in CD and 2.7% (95% CI 1.5-4.8%) in UC, indicating that UC was more often associated with PSC than CD [19].
Among the current study patients with PSC, IBD comorbidity was 100% and UC was predominant, with a comorbidity rate of 92.3%. The colonoscopy findings for all patients indicated pancolitis, and 30.8% showed rectal sparing conditions. This suggests higher IBD prevalence rates than other reports, although the rates from colonoscopy findings are considered similar to those of other reports. In addition, during the same period, among IBD patients under 18 years of age and followed at this center, PSC occurred in 5.7% of UC patients and 0.2% of CD patients, indicating that PSC comorbidity was higher among UC patients.
Our current patients were diagnosed with PSC when their serum ALP or GGT increased to at least 50% above the normal maximum values for their age group and where the ERCP or MRCP results indicated multifocal strictures, focal dilatation, or the beading of the biliary tree [15]. All of the patients included in this study also satisfied the criteria for a PSC diagnosis because their GGT in biochemical tests increased to at least 50% above the normal maximum values for their age group. However, only 15.4% of these cases showed an increased ALP. In a long-term (20-year) cohort study carried out by Feldstein et al. [4] to observe the long-term prognosis of patients with PSC, 25% of the patients diagnosed with PSC through cholangiography had normal ALP values. Because serum ALP values identify total ALP values, this suggests that when hepatic ALP isoenzyme values are measured in children, the values may be higher than the normal standard value. This variation is present because children have large personal differences depending on growth and display large ranges of normal standard values. In addition, the standard values vary with age. As a result, GGT has been suggested to be a more accurate indicator when diagnosing PSC in children [4]. Pursuant to the diagnostic recommendations of the American College of Gastroenterology (ACG) [1], a liver biopsy was not conducted when a patient met the criteria for a PSC diagnosis as a result of ERCP or MRCP findings, but was considered in cases where small duct PSC was suspected. However, all 13 patients had findings that matched a PSC diagnosis.
According to the results of long-term follow-up of PSC-IBD patients in our present study, 15.4% underwent liver transplantation due to hepatobiliary cirrhosis. In the 2003 annual report of Studies of Pediatric Liver Transplantation (SPLIT) in the United States, the ratio of child cases where patients underwent liver transplantation due to PSC was 3.5% [20]. In 2009, a study by Miloh et al. [15] showed that 19% of patients with PSC underwent liver transplantation due to repetitive cholangitis or uncompensated liver cirrhosis, and the incidence rate in that study was higher than previous reports. However, the authors of that study stated that there might have been more subjects in their hospital than in other hospitals because that institution specialized in liver treatment. To date, no treatment that can prevent the progression of liver cirrhosis has been discovered. However, since many complications of end-stage liver disease can be effectively treated, PSC should be diagnosed early, and the use of hepatotoxic drugs should be avoided. Liver cirrhosis should also be diagnosed early so that complications can be actively treated, and biliary tract cancer screening tests should also be conducted periodically [5].
Colon cancer incidence rates in PSC-UC patients are reported to be higher than in patients with UC only. The cumulative incidence rates of colon cancer in PSC-UC patients reported in Sweden were 9% at 10 years, 31% at 20 years, and 50% at 30 years; whereas, rates in UC patients were 2%, 5%, and 10%, respectively, indicating significant differences between the two conditions [21]. This was also the case in a meta-analysis reported in 2002 in the United States (odds ratio, 4.79; 95% CI, 3.58-6.41) [19]. As a result, adult PSC-UC patients are currently recommended to undergo a colonoscopy every year [72223]. Although the occurrence of IBD in pediatric patients is not an independent risk factor for colon cancer, the need for colon cancer screening tests in children has also been emphasized recently. The cumulative rate of occurrence of colon cancer among child patients diagnosed with extensive UC before 15 years of age is 40%, and disease durations are more important than the chronological ages of IBD pediatric patients [23]. Although there are currently no guidelines for periodic examinations for the early diagnosis of colon cancer in children with UC and PSC, colon cancer clearly should be diagnosed and treated early. Colon cancer occurred in 15.4% of PSC-IBD children in the present study, all of whom were UC patients. PSC comorbidity accounted for 5.7% of patients receiving follow-up due to UC in the same period.
A limitation of the present study stems from the fact that the results are technical reports prepared by a single tertiary institution. They cannot, therefore, be statistically verified and there are difficulties in generalizing the prevalence rate and progress of PSC-IBD in children in South Korea. However, these findings are important in that no previous study has reported 13 cases of children with PSC-IBD and because they included long-term follow-up results.
In conclusion, PSC in children is often associated with IBD. UC comorbidity is particularly high. Therefore, if a patient shows increases in serum GGT or ALP, or where findings in ERCP or MRCP tests indicate PSC, the patient should be evaluated for possible IBD. In the case of patients receiving follow-up due to IBD, the existence of PSC should be checked by serological tests conducted during the follow-up, and the results should be evaluated. Finally, the frequent occurrence of liver cirrhosis and colon cancer in PSC-IBD patients should be recognized and such conditions should be intensively monitored.
Figures and Tables
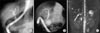 | Fig. 1(A) Endoscopic retrograde cholangiopancreatography (ERCP) showing focal dilatation and narrowing of the intrahepatic and extrahepatic bile ducts. (B) ERCP showing focal dilatation and narrowing of the intrahepatic bile ducts. (C) Magnetic resonance cholangiopancreatography revealing irregular dilatation and narrowing and beaded appearance of the intrahepatic bile duct. |
 | Fig. 2Microscopic findings showing onion-skin type periductal fibrosis and mild lymphocytic infiltration around the interlobular bile ducts (H&E stain, ×100). |
References
1. Lindor KD, Kowdley KV, Harrison ME. American College of Gastroenterology. ACG Clinical Guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015; 110:646–659. quiz 660

2. Vergani D, Mieli-Vergani G. Autoimmune hepatitis and PSC connection. Clin Liver Dis. 2008; 12:187–202. x

3. Schrumpf E, Boberg KM, Karlsen TH. Primary sclerosing cholangitis - the Norwegian experience. Scand J Gastroenterol. 2015; 50:781–796.

4. Feldstein AE, Perrault J, El-Youssif M, Lindor KD, Freese DK, Angulo P. Primary sclerosing cholangitis in children: a long-term follow-up study. Hepatology. 2003; 38:210–217.

5. Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013; 58:1392–1400.

6. Lindström L, Lapidus A, Ost A, Bergquist A. Increased risk of colorectal cancer and dysplasia in patients with Crohn's colitis and primary sclerosing cholangitis. Dis Colon Rectum. 2011; 54:1392–1397.

7. Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006; 26:31–41.

8. Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997; 41:522–525.

9. Sano H, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, et al. Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2011; 18:154–161.

10. Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007; 102:1042–1049.

11. Faubion WA Jr, Loftus EV, Sandborn WJ, Freese DK, Perrault J. Pediatric "PSC-IBD": a descriptive report of associated inflammatory bowel disease among pediatric patients with psc. J Pediatr Gastroenterol Nutr. 2001; 33:296–300.

12. Jung JA, Kwak IK, Lee HR, Jang SH, Kim KM, Yoo ES. A case of overlap syndrome with auoimmune hepatitis and cholangiopathy in a child. Korean J Pediatr Gastroenterol Nutr. 2006; 9:269–275.

13. Kim JS, Choe YH, Kim CJ, Kim WS, Kim IO, Seo JK. A case of primary sclerosing cholangitis with ulcerative colitis. J Korean Pediatr Soc. 1996; 39:1448–1454.
14. Hong J, Song MK, Ko JS, Kang GH, Kim WS, Seo JK. Autoimmune hepatitis-primary sclerosing cholangitis overlap syndrome in a 10-year-old girl with ulcerative colitis. Korean J Pediatr. 2009; 52:504–507.

15. Miloh T, Arnon R, Shneider B, Suchy F, Kerkar N. A retrospective single-center review of primary sclerosing cholangitis in children. Clin Gastroenterol Hepatol. 2009; 7:239–245.

16. MacCarty RL, LaRusso NF, Wiesner RH, Ludwig J. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983; 149:39–44.

17. Björnsson E, Boberg KM, Cullen S, Fleming K, Clausen OP, Fausa O, et al. Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut. 2002; 51:731–735.

18. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.

19. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002; 56:48–54.

20. McDiarmid SV, Anand R. SPLIT Research Group. Studies of Pediatric Liver Transplantation (SPLIT): a summary of the 2003 Annual Report. Clin Transpl. 2003; 119–130.
21. Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995; 22:1404–1408.

22. Khaderi SA, Sussman NL. Screening for malignancy in primary sclerosing cholangitis (PSC). Curr Gastroenterol Rep. 2015; 17:17.

23. Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, et al. AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010; 138:738–745.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download