Abstract
Mycoplasma pneumoniae is responsible for approximately 20% to 30% of community-acquired pneumonia, and is well known for its diverse extrapulmonary manifestations. However, acute necrotizing pancreatits is an extremely rare extrapulmonary manifestation of M. pneumoniae infection. A 6-year-old girl was admitted due to abdominal pain, vomiting, fever, and confused mentality. Acute necrotizing pancreatitis was diagnosed according to symptoms, laboratory test results, and abdominal computed tomography scans. M. pneumoniae infection was diagnosed by a 4-fold increase in antibodies to M. pneumoniae between acute and convalescent sera by particle agglutination antibody assay. No other etiologic factors or pathogens were detected. Despite the occurrence of a large infected pseudocyst during the course, the patient was able to discharge without morbidity by early aggressive supportive care. This is the first case in Korea of a child with acute necrotizing pancreatitis associated with M. pneumoniae infection.
Mycoplasma pneumoniae infection can cause a number of extrapulmonary manifestations, even in the absence of pneumonia. Extrapulmonary manifestations of M. pneumoniae infection notably involve the central nervous system, gastrointestinal tract, heart, joints, skin, and blood cells. Especially, gastrointestinal manifestations account for 25% of M. pneumoniae infections, which produce nausea, vomiting, abdominal pain, diarrhea, and loss of appetite. However, acute pancreatitis is rarely associated with M. pneumoniae infection, with scarce reports and studies in literature [12345]. We report the first case in Korea of a child with acute necrotizing pancreatitis associated with M. pneumoniae infection.
A 6-year-old girl was transferred to our hospital with a chief complaint of altered mental status. She had developed symptoms of cough and sputum 2 days before, and symptoms of epigastric pain, vomiting, and fever followed the day after. She was admitted to a secondary hospital with an impression of an unspecified viral infection. On the next day her mental status changed from alert to drowsy, and was therefore transferred to our hospital. Past medical history of the patient and family were unremarkable. Recent medication history, travel history, trauma history were all negative. Vaccination had been performed as scheduled.
Vital signs on admission showed a blood pressure of 120/73 mmHg, heart rate of 163 beats/min, respiratory rate of 44 breaths/min, and body temperature of 38.4℃. Physical examination revealed coarse breath sounds on both lung fields. Tenderness and rebound tenderness was present on the entire abdomen. No lesions were detectable on the entire body.
On neurologic examination, her mental status was drowsy with Glasgow Coma Scale scores of 13. Both pupils were isocoric with prompt light reflexes. Due to her drowsy mental status, motor strengths and sensory were uncheckable. Deep tendon reflexes were normal and pathologic reflexes of Babinski sign and ankle clonus were absent.
Initial chest and abdomen radiographs were normal. Brain computed tomography (CT) scans were unremarkable without findings of cerebral edema and hemorrhage. Initial laboratory tests revealed a hemoglobin of 14.4 g/dL, hematocrit 30.1%, white blood cell (WBC) count of 22,500/mm3, comprising 86% neutrophils, 2% lymphocytes, and 9% monocytes, and platelet count of 260×103/mm3. Electrolyte and biochemistry laboratory exams showed abnormal levels of sodium decreased to 123 mEg/L, and elevation of amylase to 1,570 U/L, lipase to 2,860 U/L, C-reactive protein (CRP) to 12.73 mg/dL, and procalcitonin to 0.54 ng/mL. Coagulation studies revealed normal activated partial thromboplastin time and fibrinogen levels, while prothrombin time was elevated to 16.9 seconds (normal range, 12.6 to 14.9 seconds), and D-dimer to 20.49 µg/mL (normal range, 0 to 0.5 µg/mL). Antithrombin III activity was decreased to 73% (normal range, 83% to 123%). Polymerase chain reaction (PCR) of respiratory viruses including adenovirus, influenza, parainfluenza, respiratory syncytial virus, metapneumovirus, rhinovirus, coronavirus were all negative. Initial serum antibody titers to M. pneumoniae detected by particle agglutination antibody assay was 1:5,120, and serum antibody titers to M. pneumoniae detected by enzyme-linked immunosorbent assay were 46.5 AU/mL for immunoglobulin (Ig) G and 1.8 index value (ratio between the absorbance value of the test sample and that of the cut-off) for IgM. Serum antibody and PCR tests to rule out other possible infectious causes, such as mumps virus, measles virus, enterovirus, herpes simplex virus, Epstein-Barr virus, cytomegalovirus, hepatitis A, hepatitis B, hepatitis C, human immunodeficiency virus, leptospira, aspergillus, and toxoplasma were all unremarkable. Abdominal CT scans revealed necrosis of the pancreas body and tail and a portal vein thrombus of 2 cm was observed in the main portal vein (Fig. 1). Bilateral pleural effusions were also observed on CT scans. Modified CT severity index was 10 (grade E, necrosis >50%), and severe acute pancreatitis was apparent according to the revised Atlanta classification [6].
The patient was admitted to the intensive care unit. She was put under fast, and sufficient fluid therapy and electrolyte correction was initiated. Intravenous gabexate mesylate and meropenem were also commenced on the first hospital day. Intravenous clarithromycin was added on the second hospital day under the presumption that her necrotizing pancreatitis was associated with M. pneumoniae infection. Her mental status gradually improved to alert during correction of fluid and electrolyte imbalances. However, fever persisted despite intravenous antibiotic therapy with meropenem and clarithromycin. Moreover, ascites and pleural effusion was observed on the third hospital day, and therefore paracentesis was performed. On the seventh hospital day, a 10×4.8×22 cm region of an infected pseudocyst was newly observed on CT scans of the abdomen (Fig. 2). A percutaneous catheter was inserted for drainage, and on the eleventh hospital day fever subsided with subsequent improvement of her general condition. Laboratory exams performed on the nineteenth day showed a WBC count of 7,000/mm3, comprising 62.8% neutrophils, 18.4% lymphocytes, and 9.3% monocytes, and platelet count of 354×103/mm3. Amylase level had decreased to 54.9 U/L, lipase to 47.2 U/L and CRP level to 0.65 mg/dL. Other laboratory tests including chemistry, electrolytes, and coagulation studies were all normal. Culture studies of the blood and peritoneal fluid that had been performed several times were all unremarkable. The patient was moved to the general ward on the fourteenth hospital day and intravenous antibiotics were stopped on the twenty-fifth hospital day.
According to serum laboratory tests performed on the twenty-second hospital day, total antibody titers to M. pneumoniae was 1:20,480, and serum antibody titers to M. pneumoniae were >100 for IgG AU/mL and 3.6 index value for IgM, confirming the diagnosis of M. pneumoniae infection (Fig. 3). Gene mutation studies were all negative for the SPINK1, PRSS1, and CFTR genes. Magnetic resonance imaging performed after one month of hospitalization revealed that the extent of the pseudocyst had decreased and that the portal vein thrombus had disappeared. However, near total necrosis of the pancreas body and tail was observed. Diet was started with supplementation of pancreatic enzymes on the thirty-second hospital day. The patient was discharged on the forty-second hospital day without any complications.
According to follow-up abdominal CT scans performed at the outpatient clinic approximately four months after discharge, the remaining pancreas was shown to constitute of the head, neck, and a small portion of the body (Fig. 4). No abnormal peripancreatic fluid collection and pseudocyst was observed. The patient is currently well without occurrence of any other complications during the first following year after discharge.
Acute pancreatitis is characterized by abdominal pain, vomiting, and elevation of pancreatic enzyme levels on laboratory tests. The incidence of acute pancreatitis in children has increased significantly in the past two decades. It is estimated that 2 to 13 new cases occur annually per 100,000 children in the pediatric population [7]. The major etiologic factors of acute pancreatitis are biliary diseases, complications from medications, systemic diseases, systemic infections, trauma, and organ transplant in the pediatric population [891011]. Although infectious causes are quite rare in the development of acute pancreatitis, mumps virus, measles virus, coxsackievirus, echovirus, rotavirus, influenza virus, Epstein-Barr virus, Mycoplasma, Salmonella, and some gram-negative bacteria are known as pathogens associated with acute pancreatitis [12].
Acute pancreatitis as an extrapulmonary manifestation related to M. pneumoniae infection has been rarely reported [12345]. Pancreatitis due to M. pneumoniae infection can range from painless pancreatitis to severe necrotizing pancreatitis, as occurred in the present case. Necrotizing pancreatitis associated with M. pneumoniae infection in the pediatric population is extremely rare with only one case reported worldwide [5]. The exact pathomechanism of M. pneumoniae infection and pancreatitis remains unknown. However, most extrapulmonary manifestations due to M. pneumoniae infection can be reasonably classified into and explained by one of the three types of the proposed pathomechanisms [3].
First, M. pneumoniae is capable of moving to distant organs hematogenously and induce the production of cytokines at the local site, causing direct-type extrapulmonary manifestations. Second, M. pneumoniae-phagocytosed macrophages are capable of presenting glycoproteins and glycolipids to the cell surface causing modulation of the immune system, leading to indirect-type extrapulmonary manifestations. Third, during the infection with M. pneumoniae, vasculitis and/or thrombosis with or without a systemic hypercoagulable state is capable of inducing vascular occlusion type extrapulmonary manifestations. Necrosis of the pancreas in our case may have been induced by vasculitis of the pancreatitis, according to the patient's status of disseminated intravascular coagulation. Moreover, portal vein thrombosis was detected on the initial abdominal CT scan. These findings suggest that the underlying pathomechanism of M. pneumoniae infection associated with acute pancreatitis may be related to the vascular occlusion type mechanism with a systemic hypercoagulable state.
In the majority of cases, acute pancreatitis does not result in severe complications in children. Severe pancreatitis is rare and is usually the result of pancreatic glandular necrosis. Necrotizing pancreatitis occurs in <1% of children with acute pancreatitis [13]. The overall mortality of acute pancreatitis is about 5% and can reach 20-30% in patients with severe acute pancreatitis and infected necrosis [1415]. A recent study has reported the variable etiology, clinical course, and outcome of acute necrotizing pancreatitis in seven children [11]. Etiologic factors-were medications, diabetes, and gallstones. All seven patients required prolonged hospitalization and five-patients required admission to the pediatric intensive care unit. All patients received aggressive supportive medical therapy, and none required surgery. There were no deaths but late complications after hospital discharge occurred in five patients which included pseudocysts, transient hyperglycemia, diabetes, and pancreatic exocrine insufficiency. The results of this study and our case highlights the need for aggressive supportive medical therapy and prolonged hospital stay for children with acute necrotizing pancreatitis. Also, close follow-up is required for the detection of possible delayed complications.
The basic concept of treatment in acute necrotizing pancreatitis is not greatly different from that of acute pancreatitis without necrosis. Restriction of oral feeding, adequate supplementation of fluid and electrolytes, enzyme inhibition therapy, pain relievers, and infection prevention are fundamentally required. Rapid and accurate initial assessment of the severity of necrotizing pancreatitis is also important, as some patients should be managed in an intensive care unit and may need a longer hospital stay. In patients with severely infected pancreatic necrosis, surgical drainage and pancreatectomy may be indicated [121617]. According to a recent report, treatment with gabexate mesilate infused continuously via catheterization of a regional artery of the pancreas was effective in a pediatric case of acute necrotizing pancreatitis caused by M. pneumoniae infection [5]. Gabexate mesilate is a synthetic serine protease inhibitor and is used to prevent or treat acute pancreatitis [18]. In an animal study, it was shown to decrease serum lipase levels, reduce the severity of the pancreatic pathology, and improve pancreatic microcirculation in rats [19]. According to the results of a meta-anlaysis, gabexate mesilate was shown to only reduce the mortality in patients with moderate to severe pancreatitis [20]. Additionally, it has been suggested that the efficacy of gabexate mesilate may differ according to the route of administration, such as intravenous or arterial infusion. The effect of early antibiotics and gabexate mesilate in our case is controversial. Despite early treatment with antibiotics and gabexate mesilate, fever persisted and an infected huge pseudocyst developed. Improvement of fever and laboratory exams were observed only after a percutaneous catheter was inserted for drainage. Therefore, close observation of possible complications and appropriate management according to the patient's clinical status is substantial in the treatment of necrotizing pancreatitis, adjoined to early primary treatments.
In summary, we report a rare case of acute necrotizing pancreatitis associated with M. pneumoniae infection in a 6-year-old child. M. pneumoniae infection should be considered a cause of children who present with severe pancreatitis with a recent history of preceding respiratory symptoms. Early aggressive supportive care and close observation of complications is crucial for preventing mortalities and morbidities.
Figures and Tables
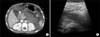 | Fig. 1(A) Initial contrast-enhanced computed tomography (CT) scans of the abdomen shows diffuse enlargement of the pancreas body and tail. Poor pancreatic parenchymal enhancement is also shown, suggesting necrosis of the pancreas and peripancreatic fluid collection with ascites. A non-occlusive thrombus in the superior mesenteric vein is also noted (white arrow). (B) Initial abdomen ultrasonography shows diffuse pancreatic swelling and low echogenicity of the total pancreas body, which correlates with the results of the abdominal CT scan. |
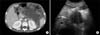 | Fig. 2(A) Follow up computed tomography (CT) scans of the abdomen conducted on the seventh hospital day shows enlarged low attenuated area with thin enhancing wall, suggestive of a pseudocyst. (B) Abdomen sonography demonstrates a 10×4.8 cm sized pseudocyst containing debris, which are consistent with the findings of the follow up CT scans. |
 | Fig. 3Serum Mycoplasma pneumoniae (MP) antibody (Ab) titers of the patient. HD: hospital day, IgG: immunoglobulin G. |
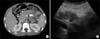 | Fig. 4(A) Computed tomography scans of the abdomen, which were conducted 4 months after discharge show normal enhancement of the remaining pancreas without pancreatic duct dilatation (small arrows). No abnormal peripancreatic fluid collection and pseudocyst was observed. The thrombus initially observed in the superior mesenteric vein (SMV) is not demonstrated (large arrow). (B) Abdominal ultrasonography, which was conducted 6 months after discharge show normal parenchymal echogenicity of the remnant pancreas without ascites or SMV thrombus. |
References
1. Mårdh PA, Ursing B. The occurrence of acute pancreatitis in Mycoplasma pneumoniae infection. Scand J Infect Dis. 1974; 6:167–171.

2. Freeman R, McMahon MJ. Acute pancreatitis and serological evidence of infection with Mycoplasma pneumoniae. Gut. 1978; 19:367–370.

3. Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010; 16:162–169.

4. al-Abassi A. Acute pancreatitis associated with Mycoplasma pneumoniae: a case report of missed diagnosis. Med Princ Pract. 2002; 11:112–115.

5. Nakagawa M, Ogino H, Shimohira M, Hara M, Shibamoto Y. Continuous regional arterial infusion therapy for acute necrotizing pancreatitis due to Mycoplasma pneumoniae infection in a child. Cardiovasc Intervent Radiol. 2009; 32:581–584.

6. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.

7. Morinville VD, Barmada MM, Lowe ME. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas. 2010; 39:5–8.

8. Benifla M, Weizman Z. Acute pancreatitis in childhood: analysis of literature data. J Clin Gastroenterol. 2003; 37:169–172.
9. Werlin SL, Kugathasan S, Frautschy BC. Pancreatitis in children. J Pediatr Gastroenterol Nutr. 2003; 37:591–595.

10. Lautz TB, Chin AC, Radhakrishnan J. Acute pancreatitis in children: spectrum of disease and predictors of severity. J Pediatr Surg. 2011; 46:1144–1149.

11. Raizner A, Phatak UP, Baker K, Patel MG, Husain SZ, Pashankar DS. Acute necrotizing pancreatitis in children. J Pediatr. 2013; 162:788–792.

12. Suzuki M, Sai JK, Shimizu T. Acute pancreatitis in children and adolescents. World J Gastrointest Pathophysiol. 2014; 5:416–426.

13. Whitcomb DC, Lowe ME. Pancreatitis: acute and chronic. In : Walker WA, editor. Pediatric gastrointestinal disease: pathophysiology, diagnosis, management. 4th ed. Hamilton: BC Decker;2004. p. 1584–1597.
14. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13:818–829.
15. Pavlidis P, Crichton S, Lemmich Smith J, Morrison D, Atkinson S, Wyncoll D, et al. Improved outcome of severe acute pancreatitis in the intensive care unit. Crit Care Res Pract. 2013; 2013:897107.

16. Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, et al. Treatment strategy against infection: clinical outcome of continuous regional arterial infusion, enteral nutrition, and surgery in severe acute pancreatitis. J Gastroenterol. 2007; 42:681–689.

17. Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014; 20:13879–13892.

18. Chen CC, Wang SS, Lee FY. Action of antiproteases on the inflammatory response in acute pancreatitis. JOP. 2007; 8:4 Suppl. 488–494.
19. Chen CC, Wang SS, Tsay SH, Lee FY, Lu RH, Chang FY, et al. Effects of gabexate mesilate on serum inflammatory cytokines in rats with acute necrotizing pancreatitis. Cytokine. 2006; 33:95–99.

20. Seta T, Noguchi Y, Shimada T, Shikata S, Fukui T. Treatment of acute pancreatitis with protease inhibitors: a meta-analysis. Eur J Gastroenterol Hepatol. 2004; 16:1287–1293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download