Abstract
Hepatocellular adenomas are a benign, focal, hepatic neoplasm that have been divided into four subtypes according to the genetic and pathological features. The β-catenin activated subtype accounts for 10-15% of all hepatocellular adenomas and specific magnetic resonance imaging features have been defined for different hepatocellular adenomas subtypes. The current study aimed to report the magnetic resonance imaging features of a well differentiated hepatocellular carcinoma that developed on the basis of β-catenin activated hepatocellular adenomas in a child. In this case, atypical diffuse steatosis was determined in the lesion. In the literature, diffuse steatosis, which is defined as a feature of the hepatocyte nuclear factor-1α-inactivated hepatocellular adenomas subtype, has not been previously reported in any β-catenin activated hepatocellular adenomas case. Interlacing magnetic resonance imaging findings between subtypes show that there are still many mysteries about this topic and larger studies are warranted.
Hepatocellular adenomas (HCA) is a benign, rare pediatric hepatic neoplasm [1]. Recently, HCA has been divided into four subtypes according to genetic and pathological features. Approximately 50% of the HCA cases are inflammatory, 35-40% are in hepatocyte nuclear factor-1α (HNF-1α)-inactivated subtype, 10-15% are in β-catenin activated and less than 10% are categorized in the unclassified subtype [2]. Specific magnetic resonance imaging (MRI) features have been defined for different HCA subtypes [23]. Herein, the current study aimed to report the MRI features of a well differentiated hepatocellular carcinoma (HCC) that developed on the basis of β-catenin activated HCA in a child. In this case, atypical diffuse steatosis was detected in the lesion. In literature, diffuse steatosis, which is defined as the feature of HNF-1α-inactivated HCA subtype, has not been previously reported in any β-catenin activated HCA case [2]. To the best of our knowledge, only a few case ware described in literature containing internal fat deposits [456].
A 15-year-old female patient was admitted to the hospital with right flank pain. She also had symptoms of sweating, weakness, and lost 2 kg of body weight. Her body mass index was 18,3 kg/m2 and family history was unremarkable. Upon physical examination, liver was palpable 6 cm under the rib and other findings were normal. There were no known diseases or drug use in the patient's history. Hepatitis B and C blood markers were negative.
Laboratory data showed marked elevation of transaminase levels as 114 IU/L aspartate aminotransferase, 112 IU/L alanine transaminase with a gamma-glutamyl transpeptidase level of 124 IU/L. Tumor marker levels were alpha-fetoprotein 15,308 µg/L and β human chorionic gonadotropin <0.1 IU/L. Other laboratory tests were unremarkable.
Abdominal ultrasound (USG) revealed a lobulated, heterogeneous, hyperechoic mass with a diameter of 14 cm in the right lobe of the liver. In computed tomography (CT), a heterogeneous, hypodense, fatty, lobulated huge mass was present, which enhanced heterogeneously. The density of tumor was measured -37 hounsfield unit in post-contrast image (Fig. 1).
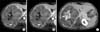
The abdominal MRI revealed a lobulated liver lesion, affecting V and VIsegments which was measured 14×15×18 cm. The lesion was hyperintense in axial in-phase gradient echo sequences (GRE) T1-weighted images (T1WI) while in out-of-phase chemical shift GRE T1WI lesion demonstrated signal drop out confirming intracellular lipid content (Fig. 2). The lesion was hyperintense in coronal T2WI (Fig. 3A) and contained a hyperintense central scar with indefinite septate formations in fat saturated T2WI (Fig. 3B). In contrast-enhanced study with gadobenate dimeglumine (Gd-BOPTA), the lesion had slightly heterogeneous contrast enhancement in the arterial phase prominent in septates. It was also noted that the contrast enhancement in the septates became more evident in portal venous phase. A long side with septate enhancement, the central scar was considerably enhanced in the hepatobiliary phase taken at the first hour (Fig. 4). Lipid containing areas of the tumor did not show significant contrast enhancement throughout all phases. Contast wash-out of tumor wasn't detected in any phase.
The needle biopsy of the tumor was evaluated as well differentiated hepato-cellular lesion; however, the differentiation of hepatic adenoma and well differentiated HCC could not be made.
The patient was evaluated for surgical resection however due to tumor size, close relation to vena cava inferior and presence of metastatic nodules in the lung parenchyma, it was not considered suitable for operation. Therefore the patient underwent chemotheraphy prior to surgery. The chemotheraphy regimen was cisplatin (80 mg/m2) for one day, adriamycin (30 mg/m2) for two days and sorafenib (250 mg/m2) 5-21 days and each regimen was repeated four times in every three weeks. After the chemotherapytumor size was regresed to 12×9×7 cm and pulmonary nodules were stable.
Thus the lesion was totally excised. The macroscopic evaluation of the right hepatectomy material revealed tumoral lesion, measuring 13×9×6 cm, with irregular borders, showing nodular growth patterns, and numerous large and small nodulations in cut surface were observed (Fig. 5A). Microscopically, the tumor was separated into nodule formations with fibrous septae and contained hemorrhagic areas, secondary to the treatment, cholesterol clefts and dense histiocytic areas. The tumor contained pleomorphic cells with large, transparent cytoplasm and hyperchromatic nuclei showing prominent nucleoli (Fig. 5B). In the immunohistochemical evaluation, the tumor cells were positive for HCC, glypican 3, CD10, and carcinoembryonic antigen. There was a focal positivity with beta catenin. With these findings, the lesion was regarded as well a differentiated HCC.
During two years follow up no relaps or distant metastasis was detected.
Although HCA is quite rare in the pediatric age group, it has been reported in girls older than 10 years of age, as in the current case [1]. They are generally asymptomatic but the patients may have the symptoms of right upper quadrant fullness or severe pain due to hemorrhage or infarction [7].
HCA has been divided into four subtypes according to genetic and pathological features. Each HCA subtype has specific gene mutations, and histomorphologic and radiologic features [7].
β-catenin activated HCA is 10-15% of all HCA [1]. Glycogen storage disease, male hormone administration, and familial polyposis syndrome are risk factors for β-catenin activated HCA development [8]. However, in the current case there were not any underlying pathology or drug usage history.
As different specific histomorphologic and radiologic features, HCA subtypes also have different risk factors and biologic behavior [7]. The risk of HCC development is defined as 7% in HNF-1α inactivated subtype, while this ratio is 46% in the β-catenin activated HCA which has highest malignancy potential [9]. Moreover, this subtype is defined as a borderline tumor between HCA and HCC [8]. In this case the tumor was a well differentiated hepatocellular lesion which was between HCA and well differentiated HCC.
In the serial of van Aalten et al. [2], the intratumoral lipid was not determined in any of the 4 β-catenin activated HCA cases. In three of the four cases, there was a T2WI hyperintense central scar. The lesions were iso-intense in T1WI, isointense-slight hyperintense in T2WI showing arterial enhancement [2]. In the current case, the findings were similar and a hyperintense central scar, which was determined with a 75% ratio of β-catenin activated HCA cases of van Aalten et al. [2], was also present in the current case.
USG, and multi-detector CT are helpful in the diagnosis of HCA. In the determination of subtypes, some MRI features have been defined [27].
In this case due to the presence of abundant intracellular lipids, in out of phase images, a profuse depression was present in the lesion. In the dynamic evaluation performed with Gd-BOPTA, the contrasting of lesion was considerably mild in the early arterial phase, while it was more prominent and heterogeneous in portal venous phase. In the hepatobiliary phase, the lesion was hyper-intense compared to the liver with a significantly enhancing central scar.
Marked intratumoral steatosis has been reported at a ratio of 78% in the HNF-1a-inactivated HCA subtype, while in 17% of inflammatory HCA mild to moderate steatosis was described by van Aalten et al. [2]. Intracellular lipids were not present in any of the β-catenin activated HCA cases [2]. However, a β-catenin activated HCA case containing focal fatty areas and showing malign transformation was reported later [256].
The presence and amount of lipid content of the tumor are highly determinant in the differential diagnosis and management. van Aalten et al. [2] suggested that steatotic HCAs less than 5 cm can be followed, but in the presence of a central scar, the lesion should be followed closely or resected.
Although histopathological evaluation is essential for the exact diagnosis, MRI findings are also important in the determination of subtypes, management of patient, and the detection of possible complications [2].
Herein, the current study reported the MRI findings of a child with β-catenin activated HCA and well differentiated HCC. The lesion was atypical with its diffuse intracellular lipid content. Interlacing MRI findings between subtypes show that there are still many mysteries about this topic and larger studies are warranted.
Figures and Tables
Fig. 1
A large fatty mass (arrow) was observed in right liver lobe in contrasted axial computed tomography. The hounsfield unit of the central point circle was -37.
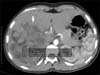
Fig. 2
(A) A hypo-intense central scar (arrow) was observed in a heterogeneous, mild hyperintense lesion in the T1 axial plan of abdominal magnetic resonance imaging and (B) there was an abundant depression of the lesion in out of phase images (arrow).
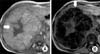
Fig. 3
In the T2-weighted (A) coronal and (B) axial images, a central scar (arrow) present in the lesion.
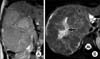
Fig. 4
(A) In the dynamic evaluation, a slightly heterogeneous contrasting lesion was present in the arterial phase prominent in septates (arrow), (B) in portal venous phase enhancement in the septates became more evident (arrow), and (C) a central scar was considerably contrasted in the late phase taken at the first hour (arrow).
References
1. Chung EM, Cube R, Lewis RB, Conran RM. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010; 30:801–826.

2. van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, et al. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011; 261:172–181.

3. Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008; 48:808–818.

4. Yoneda N, Matsui O, Kitao A, Kozaka K, Gabata T, Sasaki M, et al. Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol. 2012; 30:777–782.

6. Manichon AF, Bancel B, Durieux-Millon M, Ducerf C, Mabrut JY, Lepogam MA, et al. Hepatocellular adenoma: evaluation with contrast-enhanced ultrasound and MRI and correlation with pathologic and phenotypic classification in 26 lesions. HPB Surg. 2012; 2012:418745.

7. Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011; 258:673–693.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download