Abstract
Purpose
Gallbladder (GB) wall thickening can be found in various conditions unrelated to intrinsic GB disease. We investigated the predisposing etiologies and the outcome of acalculous GB wall thickening in children.
Methods
We retrospectively analyzed 67 children with acalculous GB wall thickening who had visited our institute from June 2010 to June 2013. GB wall thickening was defined as a GB wall diameter >3.5 mm on abdominal ultrasound examination or computed tomography. Underlying diseases associated with GB wall thickening, treatment, and outcomes were studied.
Results
There were 36 boys and 31 girls (mean age, 8.5±4.8 years [range, 7 months-16 years]). Systemic infection in 24 patients (35.8%), acute hepatitis in 18 (26.9%), systemic disease in 11 (16.4%), hemophagocytic lymphohistiocytosis in 4 (6.0%), acute pancreatitis in 3 (4.5%), and specific liver disease in 3 (4.5%) predisposed patients to GB wall thickening. Systemic infections were caused by bacteria in 10 patients (41.7%), viruses in 5 patients (20.8%), and fungi in 2 patients (8.3%). Systemic diseases observed were systemic lupus erythematosus in 2, drug-induced hypersensitivity in 2, congestive heart failure in 2, renal disorder in 2. Sixty-one patients (91.0%) received symptomatic treatments or treatment for underlying diseases. Five patients (7.5%) died from underlying diseases. Cholecystectomy was performed in 3 patients during treatment of the underlying disease.
A normal gallbladder (GB) wall appears as a pencil-thin echogenic line on an ultrasound (US) scan and is usually visible as a thin rim of soft tissue density on a computed tomography (CT) scan that enhances after contrast injection [1]. Several conditions can increase the thickness of the wall of the GB, although GB wall thickness rarely exceeds 3 mm in such cases [1]. Diffuse thickening of the GB wall may occur in patients who do not have a primary GB disease but in whom the GB is secondarily involved in an extrinsic pathologic condition. The cause of GB wall thickening can be determined in most cases by correlating the clinical presentation and associated imaging findings [1].
Acute acalculous cholecystitis (AAC) is a rare disease in children, and its spectrum has not been well established [2,3]. Thirty to fifty percent of acute cholecystitis cases in children are the acalculous type [4,5]. However, most of the previous reports related to AAC only included primary GB disease and secondary GB involvement, but most of the patients in those reports had secondary GB involvement [2,3,4,6].
The clinical and laboratory diagnoses of AAC is difficult, and the reliability of various diagnostic imaging techniques has not been established [6]. In the literature, no clear consensus appears concerning the sonographic criteria for AAC. Most studies have emphasized the combination of 1 or more sonographic findings, except those involving GB wall thickening such as hydropic dilatation of the GB, a positive sonographic Murphy sign, pericholecystic fat inflammation or fluid, or hyperemia of the GB wall at power Doppler imaging in the proper clinical setting (e.g., fever, right upper quadrant pain, and tenderness) [1,7].
Diffuse GB wall thickening in children has been found to be associated with intercurrent infection, metabolic disorders, vascular problems, trauma, and malignant disease [4,8]. In addition, it has been found in total parenteral nutrition, postoperative, posttraumatic, and extensive burn patients [9,10,11]. In these patients, a cholecystectomy is unwarranted, and the GB wall thickening usually returns to normal after the extrinsic cause is corrected [1].
Despite reports warning about the nonspecificity of GB wall thickening in children, a thick GB wall is sometimes wrongly regarded as a sign of active GB disease. Few studies have reported that GB wall thickening recovers fully with nonoperative treatment in children [3,12]. In addition, the etiology, clinical manifestations, and outcomes of acalculous GB wall thickening in children have not been evaluated in Korea. In this study, we investigated the predisposing etiologies and the outcome of acalculous GB wall thickening in children.
This study was a retrospective case analysis of 67 children who were diagnosed with acalculous GB wall thickening from June 2010 to June 2013 at Pusan National University Children's Hospital. GB wall thickening was defined as a thickening >3.5 mm on an abdominal US or a CT scan (Fig. 1).
The clinical information of patients obtained through chart review included chief complaints, laboratory findings, predisposing etiology of GB wall thickening, US or CT findings, treatment, and outcomes. The study was performed in accordance with the Declaration of Helsinki.
The GB was examined with real-time ultrasonography using 3.5- and 7.5-MHz curved array transducers and 10-MHz linear transducers of the Sequoia 512 US system (Acuson, Mountain View, CA, USA). All patients were asked to fast for 8 h before examination. Longitudinal or intercostal sonographic scans were used to measure the thickness of the GB wall. The results of abdominal US studies were retrospectively reviewed in consensus by 1 pediatric radiologist and 1 pediatric gastroenterologist. The reviewers were blinded to the clinical and laboratory features. The maximum thickness was selected. GB wall thickening was defined as a thickness of the GB wall >3.5 mm. Any other abnormalities identified on US images, including pericholecystic fluid, emphysematous GB, sludge, cholelithiasis, and hydrops, were recorded.
CT of the abdomen and pelvis were performed on 64-slice multidetector GE scanners (GE Healthcare, Milwaukee, WI, USA) or a 128-slice multidetector Siemens scanner (Siemens Medical Solutions, Forchheim, Germany). A contrast agent, iopromide (Ultravist 370; Schering, Berlin, Germany) 1.5 mL/kg of body weight, was administered intravenously at the rate of 1.5 to 2 mL/s in all children. The CT scanning protocol consisted of a 2- to 2.5-mm section thickness and a 2- to 2.5-mm reconstruction interval. Images were acquired in the portal venous phase at a 65-s delay after the start of injection. Milliamperage settings were adjusted according to patient size. The range of milliamperage settings for the patients in this study was 200 to 400 mA. Kilovoltage was held at 80 to 100 kilovolt (peak) for all patients. One pediatric radiologist retrospectively reviewed the CT findings. The reviewer was blinded to the clinical and laboratory features. The CT images were evaluated regarding the presence of diffuse GB wall thickening. The combined interpretation of axial and coronal images was performed. The presence of diffuse GB wall thickening was defined as >90% of the GB wall exceeding 3.5 mm in thickness.
A total of 67 patients were included in this study. The mean age was 8.5±4.8 years. The ages ranged from 7 months to 16 years. Of the 67 patients, 36 (53.7%) were boys, and 31 (46.3%) were girls. The male-to-female ratio was 1.15 : 1. Thirty-seven patients (55.2%) were diagnosed using abdominal US, and 30 (44.8%) were diagnosed using abdominal CT imaging. Systemic infection (24 patients [35.8%]) was the most common predisposing etiology of GB wall thickening followed by acute hepatitis (18 patients [26.9%]), systemic disease (11 patients [16.4%]), hemophagocytic lymphohistiocytosis (4 patients [6.0%]), acute pancreatitis (3 patients [4.5%]), and specific liver disease (3 patients [4.5%]) (Table 1).
Among 24 patients with systemic infection, the most common causative agent was bacteria (10 patients [41.7%]) followed by virus (5 patients [20.8%]) and fungus (2 patients [8.3%]). The causative agents could not be determined in 7 patients (29.2%). The etiologic agents of systemic infection were Salmonella typhi and Streptococcus pyogenes in 2 patients respectively, and infection with Acinetobacter baumannii, Streptococcus pneumoniae, Staphylococcus epidermidis, Klebsiella pneumonia, Burkholderia cepacia, Stenotrophomonas maltophilia in 1 patient each. The etiologic agents of systemic viral infection were Epstein-Barr virus in 4 patients and parvovirus in 1 patient. Candida krusei and Aspergillus fumigatus were the etiologic agents of fungal infection.
Among 18 patients with acute hepatitis, the etiology was unknown in cases of viral hepatitis in 15 patients (83.3%), toxic hepatitis in 2 patients (11.1%), and hepatitis A in 1 patient (5.6%).
Systemic disease included systemic lupus erythematosus in 2 patients, drug-induced hypersensitivity in 2, congestive heart failure in 2 (restrictive cardiomyopathy in 1 and pericarditis in 1), renal disorder in 2 (nephrotic syndrome in 1 and acute renal failure in 1), adult respiratory distress syndrome in 1, intestinal lymphangiectasia in 1, and asphyxia in 1 (Table 2).
Sixty-one patients (91.0%) received symptomatic treatments or treatment for underlying diseases. Five patients (7.5%) died from underlying diseases, including sepsis, hypoxic ischemic encephalopathy, diabetes mellitus, acute myelocytic leukemia, and pneumonia. Cholecystectomy was only performed in 3 patients during treatment of the underlying disease (2 liver transplantations because of fulminant hepatitis and 1 choledochal cyst). One patient was not followed up.
On the basis of the results of this study, diffuse GB wall thickening in children is caused by a wide range of extracholecystic conditions, a finding similar to previously reported studies [1,2,3,13]. Systemic infection was the most common predisposing etiology of GB wall thickening, and various etiologic agents were involved in the development of GB wall thickening. Among systemic infection, infectious mononucleosis has been reported as 1 of the major causes of GB wall thickening [14]. In our study, 4 patients had infectious mononucleosis.
Systemic diseases such as liver dysfunction, heart failure, and kidney failure may lead to diffuse GB thickening [15]. In our study, various systemic diseases were also involved in the development of GB wall thickening. Two patients with heart failure, 1 patient with liver dysfunction, and 1 patient with kidney failure were included. The exact pathophysiologic mechanism underlying edema of the GB wall in these diverse conditions is uncertain, but it is likely due to elevated portal venous pressure, elevated systemic venous pressure, decreased intravascular osmotic pressure, or a combination of these factors [1]. Hypoproteinemia has also been reported as a cause of extrinsic GB disease [1]. The accumulation of fluid within the wall may explain the thickened wall in hypoalbuminemic states. Because the subserosal layer of the GB wall contains the most areolar tissue, it is likely that fluid accumulates at this site [13]. In our study, 1 case each of nephrotic syndrome and intestinal lymphangiectasia were included.
Extracholecystic inflammation may secondarily involve the GB, thereby causing wall thickening; that occurs because of the direct spread of primary inflammation or an immunologic reaction [16]. GB wall thickening may be caused by any inflammation that extends to the GB, but only a few etiologies are regularly encountered, including hepatitis, pancreatitis, and pyelonephritis [1]. In our study, 18 patients with acute hepatitis and 3 patients with acute pancreatitis were included.
The incidence of GB wall thickening in patients with acute hepatitis was reported to be 51-90% [17,18,19]. The most common cause of acute hepatitis in our study was an unknown etiology of viral hepatitis (83%). Kim et al. [20] proposed that GB wall thickening in patients with acute hepatitis is associated with prominent changes in the muscular and serosal layers. Patients with highly elevated serum liver enzyme levels are more likely to have GB wall thickening and disruption of planes between the muscular and serosal layers than patients with normal liver enzyme levels.
Schmidt et al. [21] reported that 6 of 9 patients with hemophagocytic lymphohistiocytosis had GB wall thickening on US examination within 1 week of presentation, and speculated that GB wall thickening may be reactive in the presence of hepatitis, and it may represent edema in the presence of ascites. In our study, 4 patients with hemophagocytic lymphohistiocytosis were included.
Surgical intervention is often required in patients with diffuse GB wall thickening due to extremely rare primary GB diseases in children. The authors have never experience any case of AAC requiring surgery over the past 20 years in a single center pediatric department. Surgical procedures are unwarranted in patients with secondary GB involvement, given that the wall thickness usually returns to normal after the extrinsic factor causing the change is corrected [3]. Early differential diagnosis of GB wall thickening can be difficult. Sometimes regular and short-term follow-up US may be needed, especially in cases in which primary GB disease cannot be ruled out. In our study, cholecystectomy was not performed in any patient for the treatment of GB wall thickening. No patients had clinical deterioration because of medical treatment of GB wall thickening.
There are several limitations of our study. This is a single-center retrospective study, and we defined diffuse GB wall thickening as the maximal thickness of the GB wall on US findings, which may be associated with observational variability.
In conclusion, GB wall thickening should not cause clinicians to jump to conclusions concerning cholecystitis in children. The cause of GB wall thickening should be determined by correlating the clinical presentation and the associated imaging findings. If it is attributed to the underlying disease, this disease should be treated.
Figures and Tables
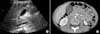 | Fig. 1An abdominal ultrasound sonography (A) and a computed tomography scan (B) showing a diffuse thickened gallbladder wall >3.5 mm in diameter. |
References
1. van Breda Vriesman AC, Engelbrecht MR, Smithuis RH, Puylaert JB. Diffuse gallbladder wall thickening: differential diagnosis. AJR Am J Roentgenol. 2007; 188:495–501.

2. Tsakayannis DE, Kozakewich HP, Lillehei CW. Acalculous cholecystitis in children. J Pediatr Surg. 1996; 31:127–130. discussion 30-1.

3. Imamoğlu M, Sarihan H, Sari A, Ahmetoğlu A. Acute acalculous cholecystitis in children: Diagnosis and treatment. J Pediatr Surg. 2002; 37:36–39.

4. Glenn F. Acute acalculous cholecystitis. Ann Surg. 1979; 189:458–465.
5. Sievert W, Vakil NB. Emergencies of the biliary tract. Gastroenterol Clin North Am. 1988; 17:245–264.

6. Mirvis SE, Vainright JR, Nelson AW, Johnston GS, Shorr R, Rodriguez A, et al. The diagnosis of acute acalculous cholecystitis: a comparison of sonography, scintigraphy, and CT. AJR Am J Roentgenol. 1986; 147:1171–1175.

7. Shetty PB, Broome DR. Sonographic analysis of gallbladder findings in Salmonella enteric fever. J Ultrasound Med. 1998; 17:231–237.

8. Lee AW, Proudfoot WH, Griffen WO Jr. Acalculous cholecystitis. Surg Gynecol Obstet. 1984; 159:33–35.
9. Weeder RS, Bashant GH, Muir RW. Acute noncalculous cholecystitis associated with severe injury. Am J Surg. 1970; 119:729–732.

10. Munster AM, Goodwin MN, Pruitt BA Jr. Acalculous cholecystitis in burned patients. Am J Surg. 1971; 122:591–593.

11. Fabian TC, Hickerson WL, Mangiante EC. Posttraumatic and postoperative acute cholecystitis. Am Surg. 1986; 52:188–192.
12. Lee SC, Tchah H, Na SY, Kim HS, Park HJ, Shin MK. Clinical and histopathologic findings on the abnormal liver function complicated with Kawasaki disease. Korean J Pediatr Gastroenterol Nutr. 2000; 3:47–55.

13. Patriquin HB, DiPietro M, Barber FE, Teele RL. Sonography of thickened gallbladder wall: causes in children. AJR Am J Roentgenol. 1983; 141:57–60.

14. Yamada K, Yamada H. Gallbladder wall thickening in mononucleosis syndromes. J Clin Ultrasound. 2001; 29:322–325.

15. Rumack CM, Wilson SR, Charboneau JW. Diagnostic ultrasound. 2nd ed. St. Louis: Mosby;1998.
16. Kaftori JK, Pery M, Green J, Gaitini D. Thickness of the gallbladder wall in patients with hypoalbuminemia: a sonographic study of patients on peritoneal dialysis. AJR Am J Roentgenol. 1987; 148:1117–1118.

17. Maudgal DP, Wansbrough-Jones MH, Joseph AE. Gallbladder abnormalities in acute infectious hepatitis. A prospective study. Dig Dis Sci. 1984; 29:257–260.
18. Giorgio A, Francica G, Amoroso P, Fico P, de Stefano G, Pierri P, et al. Morphologic and motility changes of the gallbladder in response to acute liver injury. A prospective real-time sonographic study in 255 patients with acute viral hepatitis. J Ultrasound Med. 1989; 8:499–506.

19. Suk KT, Kim CH, Baik SK, Kim MY, Park DH, Kim KH, et al. Gallbladder wall thickening in patients with acute hepatitis. J Clin Ultrasound. 2009; 37:144–148.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download