Abstract
Acute acalculous cholecystitis (AAC) is an inflammation of the gallbladder in the absence of demonstrated stones. AAC is frequently associated with severe systemic inflammation. However, the exact etiology and pathogenesis of AAC still remain unclear. Acute infection with Epstein Barr virus (EBV) in childhood is usually aymptomatic, whereas it often presents as typical infectious mononucleosis symptoms such as fever, cervical lymphadenopathy, and hepatosplenomegaly. AAC may occur during the course of acute EBV infection, which is rarely encountered in the pediatric population. AAC complicating the course of a primary EBV infection is usually associated with a favorable outcome. Most of the patients recover without any surgical treatment. Therefore, the detection of EBV in AAC would be important for prediction of better prognosis. We describe the case of a 10-year-old child who presented with AAC during the course of primary EBV infection, the first in Korea, and review the relevant literature.
Ebstein-Barr virus (EBV) infection during childhood is mainly asymptomatic, whereas infectious mononucleosis, with clinical signs such as fever, pharyngitis, lymphadenopathy, hepatosplenomegaly and hepatocellular dysfunction, occurs in at least 50% of adolescents and adults with primary infection [1]. Acute acalculous cholecystitis (AAC), an inflammation of the gallbladder in the absence of demonstrated calculi, can be an atypical clinical presentation of primary EBV infection. Approximately 5-10% of all patients with acute cholecystitis present with AAC, which is usually associated with more serious morbidity and higher mortality rates than calculous cholecystitis [2]. To date, only nine cases of AAC caused by EBV infection have been reported, although no cases have been reported in Korea [3,4,5,6,7,8,9,10,11]. We describe a case of AAC that occurred during the course of a primary EBV infection.
A 10-year-old female was admitted to Seoul National University Bundang Hospital, Korea, with a 3-day history of nausea, right upper quadrant (RUQ) abdominal pain, and a 1-day history of high fever. Before visiting our hospital, she had visited a local clinic, and abdominal ultrasonography revealed diffuse edematous wall thickening of the gallbladder. She was transferred to Seoul National University Bundang Hospital with a provisional diagnosis of acute cholecystitis and acute hepatitis. She had no history of abdominal trauma or surgery. She did not report any familial history of hepatitis or cholecystitis. On physical examination, she appeared ill. Scleral icterus was not present. The pharynx was not injected and both tonsils were not hypertrophic. The right cervical lymph node was palpable and measured approximately 2×3 cm in diameter. The abdomen was soft, not distended. Bowel sounds were normoactive. The right side of the abdomen was tender with painful fullness in the right hypochondrium, indicating a positive Murphy's sign. A 8×3 cm mass-like lesion was palpable on the RUQ of the abdomen with reddish discoloration in this area. No hepatosplenomegaly or evidence of free fluid in the abdomen was noted.
Laboratory investigations revealed a hemoglobin level of 13.7 g/dL, hematocrit value of 39.7%, platelet count of 234,000/mm3, and white blood cell count of 8,280/mm3 (neutrophils 30%, monocytes 12%, lymphocytes 56%). Atypical lymphocytes were present (1%) on peripheral blood smear. Blood chemistry revealed aspartate aminotransferase of 311 U/L, alanine aminotransferase of 489 U/L, and total/direct bilirubin level of 1.0/0.6 mg/dL. The gamma-glutamyltransferase level was raised to 308 U/L (Table 1). Other laboratory data showed a prothrombin time/international normalized ratio of 1.19, serum total protein level of 6.9 g/dL, serum albumin level of 4.5 g/dL, blood urea nitrogen level of 7 mg/dL, creatinine level of 0.54 mg/dL, and C-reactive protein level of 0.40 mg/dL.
Plain radiographys of the abdomen revealed no abnormality. Abdominal ultrasonography showed diffuse edematous wall thickening of the gallbladder (6 mm thickness) with increased vascularity. Sonographic Murphy's sign was positive. Definite echogenic calculi was not visible. Biliary ductal dilatation was not present (Fig. 1).
The following EBV panel results were indicative of acute primary infection; IgM of the viral capsid antigen (VCA) was positive (1.59, negative≤0.90, positive≥1.10). And the VCA-IgG was equivocal (0.92); the early antigen (EA) IgM was negative (5.26); and EBV EA IgG was negative (1.15). EBV EBNA IgG was negative (4.89). Other tests were performed to exclude other infectious causes of acalculous cholecystitis, the results of viral hepatitis profiles, including HBsAg, anti-hepatitis A virus IgM, anti-hepatitis C virus, and cytomegalovirus IgM were all negative.
Once the diagnosis of primary EBV infection had been confirmed, the patient was treated with empirical antibiotics, and conservative management with fasting and total parenteral nutrition. Gastrointestinal symptoms such as abdominal pain and nausea then began to improve, thus she started a regular diet on the third day of admission. She continued to have episodes of fever for approximately 5 days, but her appetite gradually improved. She eventually became afebrile for 48 hours and was discharged on the seventh day of admission. During the 2-month follow-up examination at the outpatient clinic, she was in good condition without any sign of relapse of cholecystitis.
Gallbladder disease is rare in the pediatric population. Cholecystitis during childhood and adolescence a rare, and seldom considered in the evaluation of childhood acute abdominal pain [12]. AAC is an inflammatory process of the gallbladder in the absence of gallstones, which accounts for 30-50% of pediatric cholecystitis cases [13].
The exact etiology and pathogenesis of AAC remains unclear. It is believed that acalculous cholecystitis occurs because of decreased blood flow to the gallbladder, obstruction of the biliary tract, or condensed bile [12]. In most cases, AAC in children occurs during the course of infectious diseases such as those caused by streptococci (groups A and B), Gram-negative organisms, hepatitis A virus, EBV, Leptospira interrogans, Salmonella typhi, or parasitic infestation with Ascaris or Giardia lamblia, or may result from infective endocarditis or a systemic disease such as Kawasaki disease [14]. AAC has also been reported to occur in association with abdominal trauma, extensive burns, and long-term total parenteral nutrition [14]. In addition, congenital gallbladder anomalies, congenital biliary duct anomalies and acquired disorders causing biliary stasis have been reported to be associated with childhood cholecystitis [15].
AAC caused by viral infection is extremely rare in the pediatric population. AAC complicating the course of a primary EBV infection has been seldom occurred, it is associated with a favorable outcome [11].
The clinical presentations and laboratory data of childhood AAC are nonspecific. Hence, a clinical diagnosis of AAC is difficult. Most of the patients present with fever, jaundice, RUQ or epigastric pain, nausea and vomiting. Tenderness to palpation or a mass in the RUQ is usually noted on physical examination. The pain and tenderness are less well localized to the RUQ in younger children. These symptoms and signs of AAC in children are similar to those of other intraabdominal inflammatory diseases, especially appendicitis. Ultrasonography is the main diagnostic modality for patients with AAC [16,17]. The ultrasonographic findings for acalculous cholecystitis include a gallbladder wall thickness of at least 3.5 mm, pericholecystic fluid, a sonolucent layer or halo indicative of intramural edema, and sludge or intramural gas [13,18]. A combination of at least two of the above-mentioned findings is considered to indicate AAC [19].
The treatment of AAC has been controversial. If present, underlying diseases also complicate the treatment of AAC. Treatment of AAC involves serial examinations, gallbladder ultrasonography, and cholecystectomy when indicated by deteriorating clinical or ultrasonographic findings . Accurate criteria for the timing of operative interventions have not yet been established [18]. It is acknowledged that the treatment of choice for AAC in adults is cholecystectomy [20]. However in children, initially nonoperative treatment of AAC is safe and effective in most cases [12]. Children who can be managed conservatively by active observation, nasogastric suction, and intravenous fluid and broad spectrum antibiotic administration in the early stages of the disease, will eventually recover normal gallbladder function [20]. Recently, Huang and Yang [12] reported that patients with septic shock, anemia, thrombocytopenia, hypofibrinogenemia, pericholecystic fluid, and higher ultrasonographic scores were associated with a poor outcome. Pericholecystic fluid, the most infrequent ultrasonographic finding, was often present in surgically treated patients and is highly suggestive of acute gangrenous or phlegmonous cholecystitis [18].
Our case suggests that AAC can occur during the course of acute EBV infection. The widespread use of abdominal ultrasonography in the proper clinical setting may increase the incidence of diagnosis of AAC during EBV infection compared with the previously reported incidence. Clinicians should be aware of the possible involvement of the gallbladder during EBV infection to avoid unnecessary invasive procedures or overuse of antibiotics.
Figures and Tables
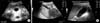 | Fig. 1Abdominal sonography taken (A) on admission, showing gallbladder wall thickening of 6 mm in a striated pattern without any definite echogenic stone, and (B) on hospital day 2, showing diffuse edematous wall thickening of the gallbladder. The hepatic parenchymal echo was normal without focal lesion. |
References
1. Macsween KF, Crawford DH. Epstein-Barr virus-recent advances. Lancet Infect Dis. 2003; 3:131–140.

2. Barie PS, Eachempati SR. Acute acalculous cholecystitis. Curr Gastroenterol Rep. 2003; 5:302–309.

3. Lagona E, Sharifi F, Voutsioti A, Mavri A, Markouri M, Attilakos A. Epstein-Barr virus infectious mononucleosis associated with acute acalculous cholecystitis. Infection. 2007; 35:118–119.

4. Prassouli A, Panagiotou J, Vakaki M, Giannatou I, Atilakos A, Garoufi A, et al. Acute acalculous cholecystitis as the initial presentation of primary Epstein-Barr virus infection. J Pediatr Surg. 2007; 42:E11–E13.

5. Iaria C, Arena L, Di Maio G, Fracassi MG, Leonardi MS, Famulari C, et al. Acute acalculous cholecystitis during the course of primary Epstein-Barr virus infection: a new case and a review of the literature. Int J Infect Dis. 2008; 12:391–395.

6. Gora-Gebka M, Liberek A, Bako W, Szarszewski A, Kamińska B, Korzon M. Acute acalculous cholecystitis of viral etiology--a rare condition in children? J Pediatr Surg. 2008; 43:e25–e27.

7. Attilakos A, Prassouli A, Hadjigeorgiou G, Lagona E, Kitsiou-Tzeli S, Galla A, et al. Acute acalculous cholecystitis in children with Epstein-Barr virus infection: a role for Gilbert's syndrome? Int J Infect Dis. 2009; 13:e161–e164.

8. Poddighe D, Cagnoli G, Mastricci N, Bruni P. Acute acalculous cholecystitis associated with severe EBV hepatitis in an immunocompetent child. BMJ Case Rep. 2014; 2014.

9. Koch AD, van den Bosch HC, Bravenboer B. Epstein-Barr virus-associated cholecystitis. Ann Intern Med. 2007; 146:826–827.

10. Cholongitas E, Katsogridakis K, Dasenaki M. Acalculous cholecystitis during the course of acute Epstein-Barr virus infection. Int J Infect Dis. 2009; 13:e129–e130.

11. Arya SO, Saini A, El-Baba M, Salimnia H, Abdel-Haq N. Epstein Barr virus-associated acute acalculous cholecystitis: a rare occurrence but favorable outcome. Clin Pediatr (Phila). 2010; 49:799–804.

12. Huang SC, Yang YJ. Septic shock and hypofibrinogenemia predict a fatal outcome in childhood acute acalculous cholecystitis. J Pediatr Gastroenterol Nutr. 2011; 53:548–552.

13. Tsakayannis DE, Kozakewich HP, Lillehei CW. Acalculous cholecystitis in children. J Pediatr Surg. 1996; 31:127–130.

14. Suchy FJ. Disease of the gallbladder. In : Kliegman RM, editor. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier;2011. p. 1415–1416.
15. Sears HF, Golden GT, Horsley JS 3rd. Cholecystitis in childhood and adolescence. Arch Surg. 1973; 106:651–653.

16. Mirvis SE, Vainright JR, Nelson AW, Johnston GS, Shorr R, Rodriguez A, et al. The diagnosis of acute acalculous cholecystitis: a comparison of sonography, scintigraphy, and CT. AJR Am J Roentgenol. 1986; 147:1171–1175.

17. Imhof M, Raunest J, Ohmann C, Röher HD. Acute acalculous cholecystitis complicating trauma: a prospective sonographic study. World J Surg. 1992; 16:1160–1165.

18. Imamogu M, Sarihan H, Sari A, Ahmetoğlu A. Acute acalculous cholecystitis in children: Diagnosis and treatment. J Pediatr Surg. 2002; 37:36–39.

19. Deitch EA, Engel JM. Acute acalculous cholecystitis. Ultrasonic diagnosis. Am J Surg. 1981; 142:290–292.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download