This article has been corrected. See "Correction: The Complex Surgical Management of the First Case of Severe Combined Immunodeficiency and Multiple Intestinal Atresias Surviving after the Fourth Year of Life" in Volume 18 on page 71.
Abstract
Severe combined immunodeficiency (SCID) is a life-threatening syndrome of recurrent infections and gastrointestinal alterations due to severe compromise of T cells and B cells. Clinically, most patients present symptoms before the age of 3 months and without intervention SCID usually results in severe infections and death by the age of 2 years. Its association with intestinal anomalies as multiple intestinal atresias (MIA) is rare and worsens the prognosis, resulting lethal. We describe the case of a four year-old boy with SCID-MIA. He presented at birth with meconium peritonitis, multiple ileal atresias and underwent several intestinal resections. A targeted Sanger sequencing revealed a homozygous 4-bp deletion (c.313ΔTATC; p.Y105fs) in tetratricopeptide repeat domain 7A (TTC7A). He experienced surgical procedures including resection and stricturoplasty. Despite parenteral nutrition-associated liver disease, the patient is surviving at the time of writing the report. Precocious immune system assessment, scrutiny of TTC7A mutations and prompt surgical procedures are crucial in the management.
Severe combined immunodeficiency with multiple intestinal atresias (SCID-MIA) is an extremely rare hereditary disease characterized by intestinal obstructions and life-threatening immune system defects involving both humoral and cellular immune systems. The genetic bases of SCID-MIA have been recently unravelled by Samuels et al. [1]: exome sequencing experiments demonstrated that the disease is caused by inactivating autosomal recessive mutation in the tetratricopeptide repeat domain 7A (TTC7A) gene, coding for a protein expressed in both thymic epithelial cells and thymocytes. TTC7A inactivation lead to severe lymphoid depletion in the thymus and peripheral lymphoid tissues from patients with SCID-MIA. Loss-of-function mutation in TTC7A was also associated with very early onset inflammatory bowel diseases [2]. Functional studies demonstrated that the mutations cause defects in enterocytes and T cells that lead to severe apoptotic enterocolitis, with or without immunodeficiency [2]. Study by Chen et al. [3] confirmed these observations in eight unrelated cases.
Our report describes the long and difficult therapeutic management of one patient among these eight that to our knowledge nowadays is the longest-surviving.
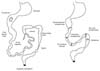
The patient was admitted to our hospital at birth, on July 2010, with diagnosis of abdominal distension and respiratory failure. The baby was born to parents of Bosnian origin, from elective caesarean section at the 34th week of gestation, with 2.710 g neonatal weight, 45 cm length, 32.4 cm head circumference, and 5/8 Apgar score at 1 and 5 minutes, respectively. His general condition at birth was poor and the baby underwent nasotracheal intubation. At a prenatal ultrasonography, intestinal perforation consistent with duodenal or jejunal atresia associated with polyhydramnios was suspected. At birth an echocardiography demonstrated a patent arterial duct with bidirectional shunting and patent foramen ovale with a negligible left to right shunt. Abdominal X-ray evidenced a clear-cut gastric air bubble, but absence of intestinal gas in all remaining abdominal fields indicating duodenal or jejunal atresia. On an emergent laparotomy, meconium peritonitis due to intestinal perforation was accompanied by 16 intestinal lesions of intestinal atresias and severe stenosis with intestinal malrotation. These traits were distanced by minimum of 3 cm and maximum of 15 cm. The sole trait without stenosis was the first jejunal loop. In addition, cecal atresia was present (Fig. 1). Serials of resection and anastomosis on three loci of ileal atresia (each one of 6, 8, and 11 cm) followed by five ileal strictureplasties were performed. Also, colic atresia and ileocecal atresia were treated with an "en bloc" resection, including cecal appendix, using a gastro-intestinal anastomosis stapler and creating an ileo-colic termino-lateral anastomosis. A colostomy at the descending colon was followed due to additional atresia at descending and sigmoid colons. The remaining ileum was estimated to be approximately 70 cm long. Histopathological examination showed the classic aspect of intestinal lumen fibrosis. In the post-operative period, parenteral nutrition (PN) was established through a central venous catheter. On August 2010 bowel obstruction due to multiple adhesions necessitated treatment with intestinal anastomosis. At the second laparotomy, tenacious adhesions were present between ileal loops and the caudate lobe of the liver that caused an obstruction of the pyloric region. Adhesiolysis of all adhesions was performed. On September 2010, a further episode of bowel obstruction occurred. At the laparotomy, tenacious adhesions that compacted all the intestinal loops to form a rigid coil were present and adhesiolysis on each lesion was performed. A CoSeal surgical sealant (Baxter Healthcare Corporation, Deerfield, IL, USA) was applied on the intestinal loop at the end of the operation for prevent spontaneous intestinal adhesion. On September 2010, we positioned a permanent central venous catheter in the left subclavian vein for long term PN. On March 2011, peritoneal adhesiolysis was necessary for a newly developed bowel occlusion. The lysis of adhesions allowed the highlighting of a discrete maturation of the intestine in terms of size and length as well as the presence of numerous ileal stenotic areas. Performing of seven enteroplasties in Heineke-Mikulizt fashion was done in correspondence at the ileal stenotic traits. We also positioned naso-jejunal two-way polyurethane enteral tube. On March 2011, dehiscence of the surgical wound occurred. Wound revision and closure with interposition of mesh was necessary. Due to infection on the abdominal wall prosthetic patch, on April 2011, it was necessary to remove the device from the abdomen and to close the abdominal wound. During the operation, under fluoroscopic control, Iomeron (Bracco Imaging SpA, Milano, Italy) was administered through the stoma, allowing the highlighting of a complete channeling of the intestine up to the duodenum and the absence spillage of the contrast agent. A radiographic intestinal transit was completed, administering contrast material through the nasogastric tube allowing an assessment of the patency of stomach, duodenum and remaining trait of the bowel.
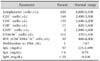
The clinical course was complicated by multiple episodes of sepsis caused by Pseudomonas aeruginosa, one with the development of an abdominal abscess, and Candida albicans. Laboratory investigations at the age of ten months showed severe T- and B-cell lymphopenia, absent proliferation to phytohemagglutinin and anti-CD3, and profound hypogammaglobulinemia (the nadir value of serum levels of immunoglobulin (Ig) G was 57, IgA and IgM values were undetectable), confirming the diagnosis of SCID (Table 1). Anti-fungal, anti-viral and anti-bacterial prophylaxes were started, together with regular intravenous immunoglobulin replacement therapy.
Whole-exome sequencing was performed on the patient and healthy family members. The analysis on the patient identified a novel deleterious variant in the gene TTC7A. Sanger sequencing to validate the deleterious variant was performed and revealed a homozygous 4-bp deletion (c.313ΔTATC) in exon 2 of TTC7A (Fig. 2). Therefore, the variant is predicted to lead to a frameshift mutation at codon 105 (p.Y105fs) and subsequent nonsense-mediated decay of the resulting ribonucleic acid transcript, which means the strong possibility of SCID-MIA.
The deletion was also present in the parents but with a heterozygote mutation and no anomaly was found in the brothers.
A multidisciplinary team meeting involving immunologist, infectious disease specialist, anaesthesiologist, pediatric surgeon and social worker was organized. The team explained to the parents the poor prognosis of their baby, and it was decided to proceed with only supportive therapies.
A percutaneous endoscopic gastrostomy was performed on September 2011 in order to decompress the stomach and small bowel and to avoid the discomfort of the nasogastric tube. In the following months the clinical improvement of the patient allowed a protected hospital discharge. The patient was discharged on May 2012 and followed-up monthly. His therapy consisted of acyclovir, fluconazole, antibiotic prophylaxis administered daily, and immunoglobulin replacement (400 mg/kg) every 21 days.
Barium follow-through x-ray via gastrostomy and colostomy at two years of age and magnetic resonance enterography at three years of age, demonstrated patency of the gut lumen and a satisfactory intestinal maturation (Fig. 3 and 4). Nevertheless, his intestines do not demonstrate adequate spontaneous motility to allow surgical recanalization in a short time, and so he lived in a status of persistent gastrointestinal sub-occlusion requiring continuously home PN with the impossibility to supply some feeding regimens via the gastrostomy tube.
In the last year the patient experienced three hospitalization, each one for a two-weeks period, presenting recurrent episodes of pancreatitis.
His neurological development and stature growth were impaired (height below the fifth percentile), but in slow progression. PN-associated liver disease was worsening progressively.
On October 2013, a multidisciplinary committee, excluded the possibility of a combined transplantation of liver and intestine due to the high risk of an aggressive form of graft-versus-host disease post-transplant [4].
Patients with SCID have recurrent and severe infections; most of these are bloodstream infections caused by intestinal bacteria, probably consequent to abnormalities of the gut barrier consistent with SCID-MIA.
Usually these patients are not able to receive enteral feeding, remain dependent on PN, have repeated episodes of sepsis, and die after a difficult neonatal intensive care course.
In our case, the patient suffering from a form of SCID with phenotype T-B-NK+, an early administered intravenous immunoglobulin replacement therapy and a most conservative resective intestinal surgery contributed to patient's survival, in spite of a persistent intestinal subocclusion status, recurrent episodes of pancreatitis and a progressive hepatopathy.
In these patients mutations of the TTC7A gene affect immune system development and function causing the association between SCID and MIA.
Most patients with SCID-MIA die early in life, and there is very limited experience with attempts to achieve systemic immunity in these cases. Few experiences were reported with HLA-matched cord blood transplantation [5]. Hematopoietic cell transplantation (HCT) from a matched unrelated donor with or without preparative chemotherapy with cyclophosphamide and thiotepa has also been attempted.
However, although the presence of T cells with a naive phenotype suggests effective de novo thymopoiesis, longer follow-up studies will be needed to confirm the efficacy of HCT [6].
Donor-derived, partial immune reconstitution has been observed after combined liver and small bowel transplantation in infants with SCID-MIA [7]. In 2004, Gilroy et al. [8] described donor immune reconstitution after a liver-small bowel transplantation for multiple intestinal atresia with immunodeficiency. At sixteen months of age, a child with SCID-MIA underwent liver-small bowel transplantation from a 1-of-6 HLA-matched donor. He achieved complete enteral autonomy and normal liver function and has never shown evidence of allograft rejection two years post-transplant. Laboratory studies revealed that more than 99% of his CD3-positive lymphocytes and 50% of his CD19-positive lymphocytes were of donor origin, whereas granulocytes and monocytes remained of recipient origin. He synthesizes polyclonal IgG, IgA, and IgM. The authors postulated that he has engrafted a donor intestine-derived immune system and is incapable of rejecting his engrafted organs.
Recently, Agarwal et al. [9] presented the clinical progress of a five year-old infant with multiple intestinal atresia and combined immunodeficiency who carries novel compound heterozygote mutations in TTC7A gene.
In conclusion, mutations in TTC7A should be scrutinized in patients with SCID-MIA. Characterization of the role of this protein in the immune system and intestinal development, as well as in thymic epithelial cells, might have important therapeutic implications in the future in SCID-MIA patients. On the other hand, SCID treatment will not provide solution to MIA.
The management of the latter will still be of surgical pertinency and surgeons must be prepared to face a broad spectrum of abdominal complications.
Precocious assessment of the immune system, scrutiny of TTC7A mutations and prompt surgical procedures are crucial in the management these patients.
Figures and Tables
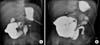
Fig. 3
Barium follow-through x-ray via gastrostomy and colostomy at 2 years of age, demonstrating patency of the gut lumen (A) and a satisfactory intestinal maturation (B).
References
1. Samuels ME, Majewski J, Alirezaie N, Fernandez I, Casals F, Patey N, et al. Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia. J Med Genet. 2013; 50:324–329.

2. Avitzur Y, Guo C, Mastropaolo LA, Bahrami E, Chen H, Zhao Z, et al. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology. 2014; 146:1028–1039.

3. Chen R, Giliani S, Lanzi G, Mias GI, Lonardi S, Dobbs K, et al. Whole-exome sequencing identifies tetratricopeptide repeat domain 7A (TTC7A) mutations for combined immunodeficiency with intestinal atresias. J Allergy Clin Immunol. 2013; 132:656–664.
4. Fischer RT, Friend B, Talmon GA, Grant WJ, Quiros-Tejeira RE, Langnas AN, et al. Intestinal transplantation in children with multiple intestinal atresias and immunodeficiency. Pediatr Transplant. 2014; 18:190–196.

5. Ali YA, Rahman S, Bhat V, Al Thani S, Ismail A, Bassiouny I. Hereditary multiple intestinal atresia (HMIA) with severe combined immunodeficiency (SCID): a case report of two siblings and review of the literature on MIA, HMIA and HMIA with immunodeficiency over the last 50 years. BMJ Case Rep. 2011; 2011. doi: 10.1136/bcr.05.2010.3031.

6. Moore SW, de Jongh G, Bouic P, Brown RA, Kirsten G. Immune deficiency in familial duodenal atresia. J Pediatr Surg. 1996; 31:1733–1735.

7. Moreno LA, Gottrand F, Turck D, Manouvrier-Hanu S, Mazingue F, Morisot C, et al. Severe combined immunodeficiency syndrome associated with autosomal recessive familial multiple gastrointestinal atresias: study of a family. Am J Med Genet. 1990; 37:143–146.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download