Abstract
Manometry is a noninvasive diagnostic tool for identifying motility dysfunction of the gastrointestinal tract. Despite the great technical advances in monitoring motility, performance of the study in pediatric patients has several limitations that should be considered during the procedure and interpretation of the test results. This article reviews the clinical applications of conventional esophageal and anorectal manometries in children by describing a technique for performing the test. This review will develop the uniformity required for the methods of performance, the parameters for measurement, and interpretation of test results that could be applied in pediatric clinical practice.
Go to : 
Manometry is used to evaluate the contractile activity of the gastrointestinal (GI) tract, which has a specific motility pattern along its segments. By measuring the pressure inside the GI tract and the pattern of the phasic contraction of the tract, problems in smooth muscle, and intrinsic or extrinsic nerves associated with GI motility may be detected.
Manometry can be performed in two methods. One is by use of a water-perfused catheter that contains multiple capillary tubes running longitudinally within it and has side holes at different levels of the longitudinal and radial axes. With a water perfusion system, pressurized water is infused through the intercapillary tubes within the catheter and the pressure change generated by the GI tract is transmitted via the radial port, through the capillary tubes, and back to the external transducers and data recorder [1]. The other method involves the use of a solid-state catheter, which does not require water or a perfusion pump. Internal microtransducers, which are pressure transducers unidirectionally and circumferentially mounted with a thin catheter, have smaller spaces between the sensors, allowing an increased number of sensors along the catheter length and detection of very rapid pressure changes compared with water-perfused catheters.
Recent technical advances in monitoring the motility pattern of GI tract involve the use of high-resolution manometry (HRM), which shows the data acquired from the solid-state catheter as pressure topography [2,3]. The larger number of sensors in the HRM provides more pressure recordings compared with conventional manometry [1]. Thus, HRM makes it possible to completely define the intraluminal pressure environment without spatial gaps between sensors and with minimal movement artifacts [1,3]. In adults, a new classification of esophageal motility has been proposed, with well-established study protocols and normative data [4]. However, a standardized protocol for performance and analysis of HRM in children has not been developed.
Performing motility studies in children has several limitations. In particular, the catheter size and water perfusion rate in children differ from those in adult standards and need to be adjusted according to the age and size of the patient. In fact, the infusion rate of the water-perfused system must be reduced to 0.02-0.25 mL/min, which is usually set as 0.1-0.4 mL/min in adults, to avoid water intoxication in small children and premature infants [5,6,7]. Since infants and toddlers usually incorporate with medical tests, the pressure tracing can be often obscured, which in turn makes the results of the manometry studies difficult to interpret when there are crying or movement artifacts. Moreover, the lack of studies in healthy children may also make the interpretation difficult and subjective. Therefore, the equipment and protocols applied to adult patients cannot be utilized successfully to test children.
This article reviews the minimum standards for conventional manometric studies in children that are suggested by a pediatric task force of the American Motility Society (AMS) [6]. Moreover, by describing a technique for performing esophageal and anorectal manometries, which are most commonly performed in the pediatric population, this document will provide some considerations regarding methods of performance, the parameters for measurement and interpretation of the test results for use in pediatric clinical practice.
Go to : 
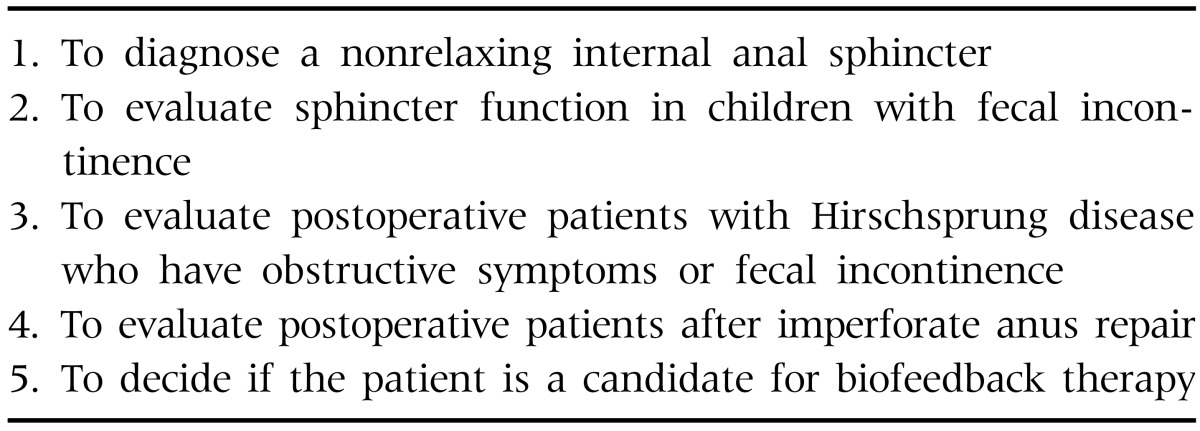
Esophageal manometry is the criterion standard for the diagnosis of primary motor disorder of the esophagus [8]. It is often performed in children with a complaint of dysphagia that is not explained by anatomical obstruction or other well-defined problems. It is not indicated in the routine evaluation of gastroesophageal reflux disease, but it can be performed in patients with an indefinite diagnosis of gastroesophageal reflux. The primary indications of esophageal manometry in children are listed in Table 1 [6,9].
Administration of medications that could affect motility, such as prokinetics, anticholinergics, and narcotics, is withheld for 48 hours prior to the procedure [9]. Patients should fast for 3 to 6 hours prior to the study (i.e., 3 hours in newborns and more than 4 hours in children) to decrease the risk of vomiting and aspiration [6]. Considering that sedation may interfere with swallowing and influence pressures, it should not be given routinely [5].
The manometric catheter is usually placed nasally in children older than 4 months, but it can be placed orally in premature infants. In older children, topical cocaine or viscous lidocaine could be used for nasal anesthesia. The patient is positioned in the supine position if a water-perfused system is used [9].
No standardized protocol has been established to perform esophageal manometry in children [9]. However, the pediatric task force of the AMS recommends performing the slow pull-through technique [6]. The catheter is withdrawn from the stomach into the esophagus in 0.5-cm steps after insertion of all the recording channels in the stomach.
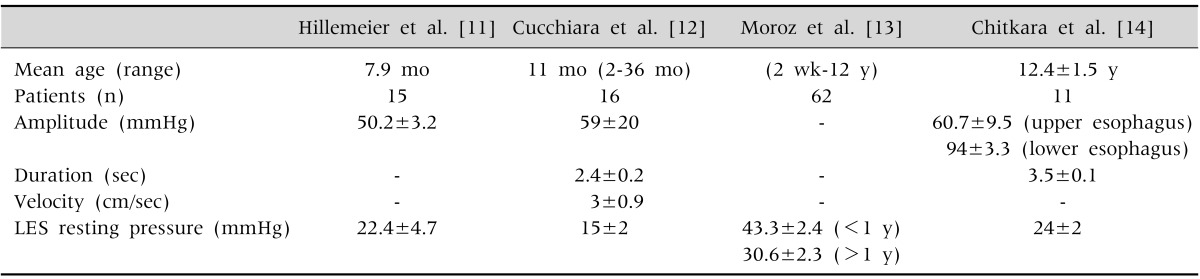
The esophagus has three functional regions, namely the upper esophageal sphincter (UES), esophageal body, and lower esophageal sphincter (LES). Both esophageal sphincters have a resting tone, which relaxes in response to swallowing, and the esophageal body has contractions that propagate [9]. By doing "wet" swallows of water at room temperature, which give a more consistent peristaltic response, the LES, amplitude, duration, and peristaltic characteristics of the contractions are measured [6].
When the all the channels are located in the stomach, the average pressure in the stomach is established as a reference baseline (a zero-value). The baseline waveform is shown as a positive pressure change with each inspiration in the channels, which verifies that the channels are located in the stomach. By performing a slow pull-through technique, the persistent rise in pressure exceeding 2 mmHg above the gastric baseline indicates that the channel has entered the LES, where the pressure is determined as the resting pressure of the LES. As the channel passes the proximal border of the LES, the pressure drops below the gastric baseline to the esophageal baseline. The overall length of the LES is defined as the distance from the first increase of >2 mmHg above the gastric baseline to the decrease to the intraesophageal baseline [5].
LES relaxation usually occurs with swallowing. The measurement of LES pressure is always performed relative to intragastric pressure. After at least one channel is placed in the stomach to show the gastric baseline, the pressure of the LES is assessed on swallowing. A bolus of water (1 mL in infants and 3-5 mL in older children) should be given to the child using a 10- to 50-mL syringe. If the patient is older than 3 years, the patient is asked to swallow. If the child is very young or uncooperative, water can be injected in the mouth while he/she is using a pacifier [5]. When the LES is completely relaxed, the pressure drops almost to the gastric baseline [5,6].
When the distal channel is placed 3 cm above the proximal border of the LES, 10 separate wet swallows are performed with a 1- to 5-mL bolus of water [5,9]. There has to be at least 20-30 seconds between each wet swallow so as not to inhibit the contractile activity.
The following four parameters are usually evaluated: (1) amplitude of the contractions, (2) duration of the contractions, (3) peristalsis (propagation rate/velocity), and (4) characteristics of the contraction waves. In general, a pressure complex in the distal esophagus with an amplitude <35 mmHg is considered hypotensive, whereas a contraction amplitude >180 mmHg is considered hypertensive [8,10]. The cutoff of 30 mmHg is now used to separate effective from ineffective peristalsis [8]. The typical duration of peristaltic contractions is approximately 4 seconds [10]. When primary peristalsis occurs after swallowing, the contractions usually progress at a speed of 2 to 4 cm/sec [9].
UES relaxation also occurs with swallowing. Because the brisk movements of the larynx and sphincter are discordant with the movement of the intraluminal recording device, UES studies have been difficult to perform and the results are not sensitive enough to have a clear impact on patient management [8]. Therefore, UES studies should be performed with a specially designed assembly [5].
Owing to a paucity of studies in normal control, normal motility patterns in children are difficult to establish, which may lead to overinterpretation. Normal values for esophageal manometry in children are presented in Table 2, which are based on a number of limited studies [11,12,13,14].
The following four manometric findings are characteristics of achalasia: (1) increased LES resting pressure, (2) incomplete or abnormal LES relaxation, (3) absence of esophageal peristalsis, (4) elevated intraesophageal pressure as compared with intragastric pressure [15,16]. Among these findings, the absence of esophageal body peristalsis is necessary for the diagnosis of achalasia, and the other criteria are often fulfilled but not required.
The characteristic manometric findings are as follows: (1) repetitive and simultaneous (nonperistaltic) contraction, at least 20% of the wet swallows; (2) periods of normal peristaltic sequences; (3) alteration in the contraction waves (repetitive and increasingduration and high amplitude), although some patients may have normal amplitudes; (4) a normal LES in most patients, although incomplete LES relaxation or a hypertensive sphincter has been described [8,17].
Of all the collagen vascular disorders, scleroderma shows the most marked esophageal abnormalities even in children [18], (1) incompetent LES, (2) low amplitude of distal esophageal contraction, and (3) weak or absent distal esophageal peristalsis.
Go to : 
Anorectal manometry represents one of the most frequently performed motility tests in children. The main indication is in the evaluation of children with constipation to exclude a nonrelaxing internal anal sphincter (IAS), which is found in IAS achalasia and Hirschsprung disease [6,19]. Anorectal manometry is also indicated in the evaluation of children with fecal incontinence to assess sphincter function and weakness, as well as sensation. The primary indications of the test in children are listed in Table 3 [6].
Before the procedure, a complete clearance of the lower bowel is needed. In older patients, a phosphate enema is usually given the night before the study. However, bowel preparations should not be performed for newborns to avoid false-positive results that suggest Hirschsprung disease [5].
Sedation for the performance of anorectal manometry may not necessary for young children. If it is needed, however, chloral hydrate and midazolam can be introduced. These medications have been reported not to affect the anal sphincter function during the procedure [6]. Children receive nothing by mouth for 4 to 6 hours before the procedure (depending on their age) if they will be sedated [9].
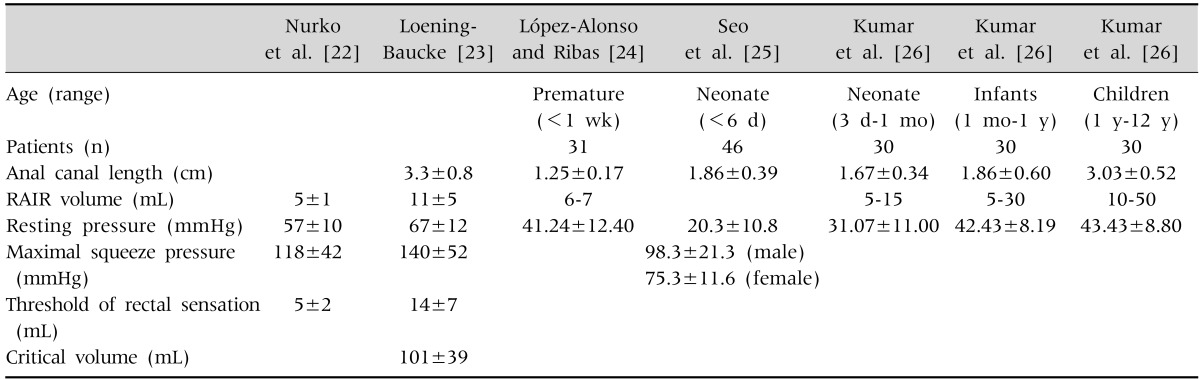
In children, a water-perfused catheter is the most commonly used in anorectal manometry, where a balloon can be attached to the distal end of the catheter for rectal distension. A balloon size of 1×1 cm or 3×5 cm is usually suggested for newborns, and toddlers and older children, respectively, when deflated and placed in the rectum [6]. The volume of the balloon required for rectal distension is usually considered to be 15 mL for newborn, 30 mL for infants, and 60 mL for older children. However, it is important to use larger balloon volumes in patients with megarectum because a lack of sphincter relaxation may result from inadequate distension of the rectal wall [6,9].
Patient should be positioned on their left side, with hips and knees flexed. After insertion of a well-lubricated manometric catheter in the anal canal, the patient is allowed time to adjust to the catheter of at least 2-10 minutes [9]. The recording probe should be correctly positioned in the anal canal and kept in place during the procedure for the anal sphincter assessment. The anal canal, which is usually considered to be 1 cm to 2 cm above the anal verge in children, is defined as the region where the pressure is increased to higher than 5 mmHg of the rectal pressure as the probe is retracted stepwise at an interval of 0.5 cm from the rectum after catheter insertion to the rectum (stationary pull-through method) [6,9].
After correct placement of the probe in the anal canal is confirmed, the resting pressure of the anal sphincter can be recorded with a stable baseline. The resting pressure of the anal canal is comprised of both internal and external anal sphincter contractions, with the former providing approximately 75% of the total pressure.
If the children are cooperative, when they are asked to squeeze as hard as possible for 10-20 seconds, the maximum pressure obtained that is higher than the anal resting pressure is measured as the maximal squeeze pressure. The pressure is determined by the conscious contraction of the external anal sphincter and puborectalis muscle, which consist of striated muscle fibers and are innervated by the somatic nerve.
When the patient is asked to cough, the external anal sphincter normally contracts in response to a sudden increase in abdominal pressure.
When the patient is asked to push as if trying to defecate, the external anal sphincter should normally relax during the maneuver. Because patients have been found to have difficulty performing this test due to embarrassment in the testing environment, this test is considered optional.
When a fecal bolus enters the rectum, there is a reflex relaxation of the IAS (rectoanal inhibitory reflex [RAIR]), which occurs independently of the spine and is lost when inhibitory ganglion cells are lacking (as is the case in Hirschsprung disease) [20]. The balloon placed in rectum is inflated to elicit the RAIR, and the characteristics of the IAS relaxation are studied. Air is rapidly injected to inflate of the balloon and then immediately withdrawn within 3-5 seconds of inflation. Repeated inflations of the balloon with an increase in volume by 10 mL each time are performed until the RAIR is obtained. The minimum amount of air required to elicit an IAS relaxation is determined. Although the suggested maximum inflation volume required for obtaining RAIR is 30 mL for infants and 60 mL for older children, it is important to use larger balloon volumes in patients with megarectum because a lack of sphincter relaxation may result from inadequate distension of the rectal wall [6,9]. In newborns, 15 mL is enough to obtain RAIR [5].
During manometric testing with a rectal balloon, some sensory information can be obtained. The balloon volume is increased for rectal distension in increments of 10 mL. After each inflation, the distension is maintained for 30 seconds and the balloon is completely deflated. At each increment of air volume injected in the rectal balloon, patients are asked if they feel a sensation of fullness or bloating or gas (first sensation) and if they have the desire or urge to defecate (desire to defecate). Critical volume (maximum tolerable volume) is defined as the minimum amount of air that produces a lasting urge to defecate.
Anorectal function during straining for defection can be evaluated with the use of electromyography (EMG) of the pelvic muscles in children older than 5 years. While EMG electrodes are attached to the external anal sphincter and buttocks, the children are asked to defecate a rectal balloon filled with 100 mL of water, while sitting on a portable toilet chair [5]. The defecation effort is evaluated by measuring the combined external and internal anal sphincter pressures, abdominal pressure exerted onto the rectum and EMG activity of the external anal sphincter and pelvic floor muscle. Normal defecation consists of increased rectal (intra-abdominal) pressure, decreased anal pressure, and decreased external anal sphincter EMG activity [6].
While normal values for anorectal manometry in adults have been published, limited information in healthy children is available [21]. The values for normal control are presented in Table 4 [22,23,24,25,26].
Fecal incontinence has various causes. However, the most common cause of fecal incontinence in children has been reported to be fecal impaction. Fecal impaction may alter the tone and viscoelastic properties of the bowel wall, consequently causing the decrease of the anorectal sensitivity and reflex inhibition of the IAS when feces enter the rectum [5]. Therefore, several different abnormalities can be observed as follows: (1) decrease in resting pressure, (2) decrease in maximum squeeze pressure, (3) decrease in maximum tolerable rectal volume, (4) reduced rectal volume necessary to induce sphincter relaxation (RAIR), (5) impaired external anal sphincter response to rectal distention and increases in intra-abdominal pressures (cough test).
Paradoxical contraction of the external anal sphincter and/or puborectalis muscle is measured while the patient strains to defecate (push pressure or defecation dynamics), which could be observed in healthy patients owing to embarrassment during the procedure in the test environment. The threshold volume required to induce an urge to defecate is usually abnormally high.
The IAS response to rectal distension is abnormal. The RAIR is absent on anorectal manometry.
False-negative results represent artifacts such as probe movements, passage of flatus or feces, or external anal sphincter relaxation [27]. To avoid errors, the rectum should be empty, the correct probe position should be ensured, and probe movements should be closely monitored [9]. False-positive results may be caused by the immaturity of ganglion cells in premature infants and full-term neonates, high relaxation threshold in some children, and technical errors in which the relaxation zone is missed [27].
Go to : 
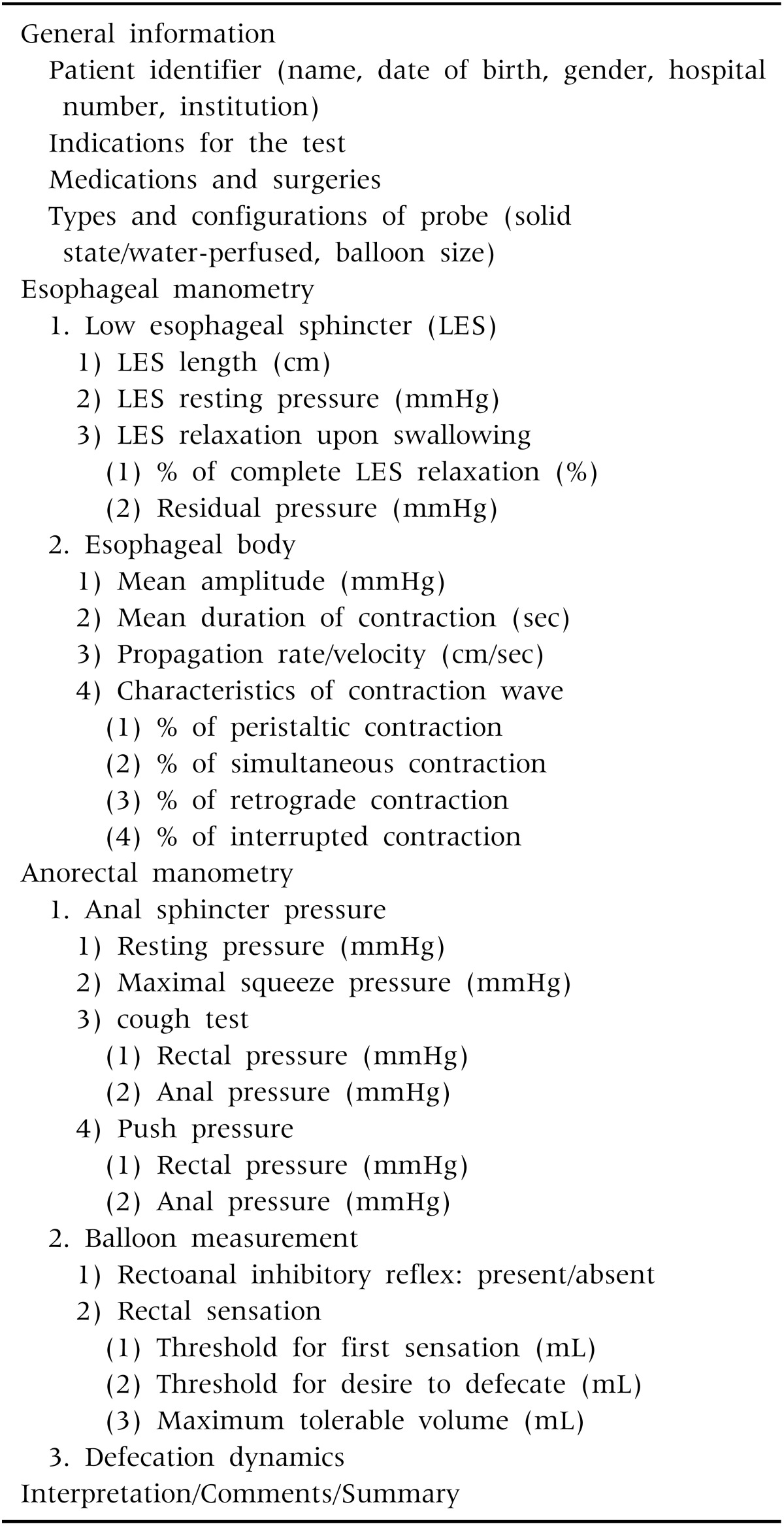
The components of the report in esophageal manometry are presented in Table 5. This article focuses on the conventional esophageal and anorectal manometric tests most commonly performed in the pediatric population, although colonic and antroduodenal tests have also been performed with increasing frequency. The pediatrician performing the test or interpreting the results should be familiar with the developmental and behavioral patterns of children, and the characteristics of the procedure and GI motility. This article provides uniform information with regard to the methods of performance and interpretation of test results and also suggests a standard template for reporting the results, which is useful in pediatric clinical practice.
Go to : 
References
1. ASGE Technology Committee. Wang A, Pleskow DK, Banerjee S, Barth BA, Bhat YM, et al. Esophageal function testing. Gastrointest Endosc. 2012; 76:231–243. PMID: 22657403.

2. Clouse RE, Prakash C. Topographic esophageal manometry: an emerging clinical and investigative approach. Dig Dis. 2000; 18:64–74. PMID: 11060469.

3. Dogan I, Mittal RK. Esophageal motor disorders: recent advances. Curr Opin Gastroenterol. 2006; 22:417–422. PMID: 16760760.

4. Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008; 103:27–37. PMID: 17900331.

5. Stendal C. Practical guide to gastrointestinal function testing. Malden: Blackwell Science;1997. p. 247–266.
6. Di Lorenzo C, Hillemeier C, Hyman P, Loening-Baucke V, Nurko S, Rosenberg A, et al. Manometry studies in children: minimum standards for procedures. Neurogastroenterol Motil. 2002; 14:411–420. PMID: 12213110.

7. Omari T, Benninga MA, Barnett CP, Haslam RR, Davidson GP, Dent J. Characterization of esophageal body and lower esophageal sphincter motor function in the very premature neonate. J Pediatr. 1999; 135:517–521. PMID: 10518089.

8. Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 1994; 107:1865–1884. PMID: 7958705.

9. Nurko S. Gastrointestinal manometry: methology and indication. In : Kleinman RE, Sanderson IR, Goulet O, Sherman PM, Mieli-Vergani G, Shneider BL, editors. Walker's pediatric gastrointestinal disease. Hamilton: BC Decker Inc.;2008. p. 1375–1392.
10. Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL 3rd, Castell JA, et al. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of "abnormal" contractions. Dig Dis Sci. 1987; 32:583–592. PMID: 3568945.
11. Hillemeier AC, Grill BB, McCallum R, Gryboski J. Esophageal and gastric motor abnormalities in gastroesophageal reflux during infancy. Gastroenterology. 1983; 84:741–746. PMID: 6825985.

12. Cucchiara S, Staiano A, Di Lorenzo C, D'Ambrosio R, Andreotti MR, Prato M, et al. Esophageal motor abnormalities in children with gastroesophageal reflux and peptic esophagitis. J Pediatr. 1986; 108:907–910. PMID: 3712155.

13. Moroz SP, Espinoza J, Cumming WA, Diamant NE. Lower esophageal sphincter function in children with and without gastroesophageal reflux. Gastroenterology. 1976; 71:236–241. PMID: 939384.

14. Chitkara DK, Fortunato C, Nurko S. Prolonged monitoring of esophageal motor function in healthy children. J Pediatr Gastroenterol Nutr. 2004; 38:192–197. PMID: 14734883.

15. Tovar JA, Prieto G, Molina M, Arana J. Esophageal function in achalasia: preoperative and postoperative manometric studies. J Pediatr Surg. 1998; 33:834–838. PMID: 9660208.

16. Katz PO, Richter JE, Cowan R, Castell DO. Apparent complete lower esophageal sphincter relaxation in achalasia. Gastroenterology. 1986; 90:978–983. PMID: 3949123.

17. Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004; 99:1011–1019. PMID: 15180718.

18. Flick JA, Boyle JT, Tuchman DN, Athreya BH, Doughty RA. Esophageal motor abnormalities in children and adolescents with scleroderma and mixed connective tissue disease. Pediatrics. 1988; 82:107–111. PMID: 3288952.

19. Ciamarra P, Nurko S, Barksdale E, Fishman S, Di Lorenzo C. Internal anal sphincter achalasia in children: clinical characteristics and treatment with Clostridium botulinum toxin. J Pediatr Gastroenterol Nutr. 2003; 37:315–319. PMID: 12960655.

20. Loening-Baucke V, Pringle KC, Ekwo EE. Anorectal manometry for the exclusion of Hirschsprung's disease in neonates. J Pediatr Gastroenterol Nutr. 1985; 4:596–603. PMID: 4032175.
21. Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999; 94:773–783. PMID: 10086665.

22. Nurko S, Garcia-Aranda JA, Guerrero VY, Worona LB. Treatment of intractable constipation in children: experience with cisapride. J Pediatr Gastroenterol Nutr. 1996; 22:38–44. PMID: 8788285.

23. Loening-Baucke V. Anorectal manometry and biofeedback training. In : Hyman PE, editor. Pediatric gastrointestinal motility disorders. New York: Academy Professional Information Systems Inc.;1994. p. 231–252.
24. López-Alonso M, Ribas J. Technical improvement for anorectal manometry in newborns. J Pediatr Surg. 1991; 26:1215–1218. PMID: 1779331.

25. Seo JM, Choi YM, Lee EH, Jun YH, Ahn SI, Hong KC, et al. Anorectal manometry in normal neonates. J Korean Assoc Pediatr Surg. 1999; 5:103–110.

26. Kumar S, Ramadan S, Gupta V, Helmy S, Atta I, Alkholy A. Manometric tests of anorectal function in 90 healthy children: a clinical study from Kuwait. J Pediatr Surg. 2009; 44:1786–1790. PMID: 19735826.

27. Meunier P, Marechal JM, Mollard P. Accuracy of the manometric diagnosis of Hirschsprung's disease. J Pediatr Surg. 1978; 13:411–415. PMID: 682091.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download