Abstract
Co-existing pyloric submucosal masses with hypertrophic pyloric stenosis (HPS) are very rare and treating these lesions is always a problem. A 20-day-old boy presented with recurrent episodes of projectile non-bilious vomiting lasting for 5 days. HPS was suspected due to the presenting age and the symptoms. The sonography demonstrated not only circumferential wall thickening of the pylorus, but also a pyloric submucosal mass. At laparotomy, a 0.8 cm sized pyloric submucosal mass was identified along with a hypertrophied pylorus. Pyloric excision was performed due to the possibility of sustaining the symptoms and malignancy. The pathological report of the submucosal mass was ectopic pancreas. Coexisting pyloric lesions can be diagnosed along with HPS, and surgical excision, not just pyloromyotomy, should be considered in these circumstances. To the best of our knowledge, this is the first case report of pyloric ectopic pancreas and HPS to be diagnosed concurrently.
Hypertrophic pyloric stenosis (HPS) is an important differential diagnosis of emesis in infants aged 2 to 12 weeks. The diagnosis is initially suggested by its unique history of projectile nonbilious vomiting after feeding. Various pyloric masses such as pyloric duplication, adenomyoma, or ectopic pancreas, have been reported to mimic HPS, being diagnosed through preoperative imagings or explorative laparotomies [1,2,3]. Besides these conditions, there have been few reports of a pyloric mass being diagnosed concurrently with HPS. Due to limitations of diagnostic modalities for neonates or small infants, preoperative pathologic diagnosis of the masses and decisions of how to treat the lesions is difficult [4,5].
We report a case of ectopic pancreas at the pylorus that was concurrently diagnosed with HPS and was treated with pyloric excision. We studied the method of treating the pyloric submucosal mass that coexisted with HPS.
A 20-day-old male infant presented with recurrent episodes of projectile non-bilious vomiting. He was vomiting about 10 times a day, especially after breast feeding for 5 days. The patient had 500 g weight reduction over these 5 days. There was no sign of dehydration at admission, as the patient had intravenous hydration at a local hospital before being transferred.
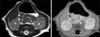
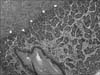
HPS was suspected due to presenting age and symptoms, so ultrasonography was done immediately. The ultrasonography revealed pyloric muscle thickness of 4 mm and a length of 16 mm and demonstrated not only HPS, but also a 0.8×0.7×0.4 cm intramural cystic pyloric submucosal mass (Fig. 1). A fluoroscopic upper gastrointestinal contrast study (UGI study) also demonstrated HPS features, narrowing and shouldering at the pylorus and severely delayed gastric emptyping, but did not show any evidence of the pyloric submucosal mass seen on the sonography (Fig. 2). Magnetic resonance imaging (MRI) was done for differential diagnosis of this submucosal mass; ectopic pancreas, adenomyoma, or duplication cyst was suspected (Fig. 3). At laparotomy, a pyloric submucosal tumor surrounded with mild inflammation was identified along with hypertrophied pylorus. To excise the pyloric submucosal tumor, pyloric excision was performed (Fig. 4). Pathological report of the submucosal tumor was ectopic pancreas and also showed a hypertrophied outer proper muscle of the pylorus, consistent with HPS (Fig. 5).
Diet was extended to full feeding with no problems and the patient was discharged on postoperative day 8. The child had no postoperative complications after 4 months follow up.
Gastric masses in children are rare and their histology is usually benign [6], but the differential diagnosis is quite diverse. Benign lesions such as gastric duplication cyst, ectopic pancreas, lipoma, hyperplastic polyp, and inflammatory fibroid polyp must be considered along with neoplastic lesions such as gastrointestinal stromal tumor, lymphoma, and carcinoid tumor. Benign gastric lesions have been reported to cause various symptoms such as, abdominal pain, pyloric stenosis, gastrointestinal bleeding, intussusceptions, and autoimmune-like symptoms [7,8,9,10].
There are several reports of various gastric lesions coexisting with HPS. A hyperplastic antral polyp obstructing the pylorus after pyloromyotomy for HPS has been reported [5] and coexisting asymptomatic gastric duplication cyst, antral webs have been reported too [4,11,12]. Gastric lesions coexisting with HPS are not rare and must not be missed when diagnosing HPS. When diagnosis of these gastric lesions is missed, the symptoms mentioned above can be presented postoperatively neecessitating a second operation. Also, although not reported yet in infants, these gastric lesions could be neoplastic and when malignancy is suspected preoperatively, pathologic confirm through surgery would be needed.
Generally, to evaluate gastric submucosal lesions, endoscopy and computed tomography are used. Especially, gastric ectopic pancreas could be diagnosed with only endoscopy by its distinguishing findings [13,14]. But, in this patient, with pyloric obstruction, performing the endoscopic examination could lead the perforation of stomach. Furthermore, because the submucosal mass was located within the hypertrophyed pylorus, endoscopy could not have reached the lesion. So, for the diagnosis of gastric lesions coexisting with HPS, ultrasonography was performed, instead of endoscopy. For evaluating the cause of obstruction and natures of mass, UGI study and abdomen MRI were conducted too, but they were not a necessity and didn't give valuable additional information. When coexisting pyloric submucosal masses and HPS is encountered, we recommend ultrasonography for screening and UGI and abdomen MRI for further evaluation.
For the treatment of these coexisting lesions there are different suggested treatments. In the case report of a hyperplastic antral polyp coexisting with HPS, the polyp was surgically excised during a second operation due to obstructing symptoms after the initial pyloromyotomy [5]. A gastric duplication cyst which was coincidentally detected during pyloromyotomy for HPS, was surgically excised at a second laparotomy at when the patient was 12 months old to avoid possible symptoms [4].
In contrast, in the several cases of antral web coexisting with HPS, there was no treatment performed for these webs, as they were not obstructing lesions [11,12]. In this patient, an 8 mm, relatively large submucosal mass was located at the middle of the 2 cm sized pylorus. If the mass was not the cause of the pyloric obstruction at this time, it would possibly be a cause in the future. We considered the pyloric excision would be be necessary to avoid a second laparotomy. The possibility of malignancy was also considered but not significantly.
In the case of an ectopic pancreas coexisting with HPS as in this case, surgical excision is controversial. As there is no evidence of an ectopic pancreas as a pre-malignant lesion, no optimal treatment has been established [15]. But an ectopic pancreas can develop various symptoms starting from common symptoms as epigastric pain [16] to gastrointestinal hemorrhage [17,18], obstructive jaundice, pyloric stenosis [3] and intussusception. Generally, the treatment of an asymptomatic ectopic pancereas is just observation, but when encountered at laparotomy, this lesion should probably be excised, unless the excision would bring significant risk of morbidity [19].
Also almost any pathologic process that may affect the normal pancreas may develop in the ectopic pancreas, including acute and chronic pancreatitis, abscess or pseudocyst formation and even malignancy [16,20]. So, many papers recommend surgical exploration for definite diagnosis, the possibility of developing symptoms, and exclusion of the possibility of malignancy [16,20]. Local excision is all that in necessary, but when the diagnosis is uncertain or malignancy is suggested, intraoperative frozen biopsy and more formal gastric resection may be needed.
In the case presented, accurate diagnosis was not possible through preoperative imaging. So for adequate diagnosis, and due to the possibility of malignancy and development of symptoms, we performed pyloric excision, rather than pyloromyotomy.
To evaluate the adverse effect of pyloric excision in an infant was difficult due to lack of previous studies. It was presumed to be possible based on comparative studies between the adult patients who conducted pylorus-preserving gastrectomy and conventional distal gastrectomy with Billroth-I reconstruction due to stomach cancer. In these studies, patients who underwent pylorus excision showed more frequent symptoms due to alkaline reflux and postprandial discomfort [21]. In this case, the patient was too young to express the symptoms and more follow-up period is needed.
This was a case report of a 20-day-old boy diagnosed of HPS and ectopic pancreas in the pylorus;to the best of our knowledge this is the first report of these two diseases presentingat the same time. For the diagnosis of pylroic submucosal lesions with HPS, ultrasonography was most the helpful test under the circumstances as endoscopy could not be conducted. And for similar patients, because of the possibility of pyloric obstructive symptoms owing to pyloric submucosal mass and the risk of malignancy, pyloric excision, including pyloric submucosal mass excision should be considered, instead of pyloromyotomy.
Figures and Tables
 | Fig. 1Abdomen ultrasonography. (A) 0.8×0.7×0.4 cm intramural cystic lesion at the anterior wall of the pylorus (arrow). (B) Hypertrophied pyloric muscle (arrow). |
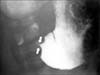 | Fig. 2Fluoroscopic upper gastrointestinal contrast study. Narrowing and shouldering at the pylorus (arrows) consistent with hypertrophic pyloric stenosis. |
References
1. Chin AC, Radhakrishnan RS, Lloyd J, Reynolds M. Pyloric duplication with communication to the pancreas in a neonate simulating hypertrophic pyloric stenosis. J Pediatr Surg. 2011; 46:1442–1444.

2. Takeyama J, Sato T, Tanaka H, Nio M. Adenomyoma of the stomach mimicking infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2007; 42:E11–E12.

3. Ozcan C, Celik A, Güçlü C, Balik E. A rare cause of gastric outlet obstruction in the newborn: Pyloric ectopic pancreas. J Pediatr Surg. 2002; 37:119–120.

4. Hishiki T, Saito T, Terui K, Mitsunaga T, Nakata M, Matsuura G, et al. A rare presentation in a case of gastric duplication cyst communicating to the pancreatic duct: coincidental detection during pyloromyotomy for hypertrophic pyloric stenosis. J Pediatr Surg. 2008; 43:e1–e3.

5. Kim S, Chung CJ, Fordham LA, Specter BB. Coexisting hyperplastic antral polyp and hypertrophic pyloric stenosis. Pediatr Radiol. 1997; 27:912–914.

6. Murphy S, Shaw K, Blanchard H. Report of three gastric tumors in children. J Pediatr Surg. 1994; 29:1202–1204.

7. Chongsrisawat V, Yimyeam P, Wisedopas N, Viravaidya D, Poovorawan Y. Unusual manifestations of gastric inflammatory fibroid polyp in a child. World J Gastroenterol. 2004; 10:460–462.

8. Thompson WM, Kende AI, Levy AD. Imaging characteristics of gastric lipomas in 16 adult and pediatric patients. AJR Am J Roentgenol. 2003; 181:981–985.

9. Iwasaki M, Nishimura A, Kamimura R, Ura K, Kobayashi H, Saiga T. Pyloric duplication cyst in an infant. Pediatr Int. 2009; 51:146–149.

10. Reggoug S, Errabih I, Ouazzani L, Benzzoubeir N, Krami H, Raiss M, et al. Heterotopic pancreas: an unusual cause of epigastric pain. Gastroenterol Clin Biol. 2010; 34:726–727.

11. Mandell GA. Association of antral diaphragms and hypertrophic pyloric stenosis. AJR Am J Roentgenol. 1978; 131:203–206.

12. Bell MJ, Ternberg JL, Keating JP, Moedjona S, McAlister W, Shackelford GD. Prepyloric gastric antral web: a puzzling epidemic. J Pediatr Surg. 1978; 13:307–313.

13. Lee SY, Ko JS. Gastric duplication cyst with ectopic pancreas in a child. Korean J Gastroenterol. 2012; 60:391–393.

14. The Information Committee of the Korean Gastric Cancer Association. 2005~2006 Nationwide gastric submucosal tumor report in Korea. J Korean Gastric Cancer Assoc. 2008; 8:104–109.
15. Ogata H, Oshio T, Ishibashi H, Takano S, Yagi M. Heterotopic pancreas in children: review of the literature and report of 12 cases. Pediatr Surg Int. 2008; 24:271–275.

16. Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol. 2007; 13:6098–6100.

17. Ueno S, Ishida H, Hayashi A, Kamagata S, Morikawa M. Heterotopic pancreas as a rare cause of gastrointestinal hemorrhage in the newborn: report of a case. Surg Today. 1993; 23:269–272.

18. Tomita S, Kang J, Ghassemi M. Heterotopic pancreas-an unusual cause of melena in a pediatric patient. J Pediatr Surg. 2009; 44:2432–2433.

19. Malek MM, Gittes GK. Lesions of the pancreas. In : Holcomb GW, Murphy JP, editors. Ashcraft's pediatric surgery. 5th ed. Philadelphia: Elsevier Saunders;2010. p. 605–615.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download