Abstract
Lymphocytic gastritis (LG) is a rare subtype of chronic gastritis. It is defined as dense proliferation of intraepithelial lymphocytes (IELs) more than 25 lymphocytes per 100 epithelial cells. The known major causes of LG are celiac disease and Helicobacter pylori infection. H. pylori associated LG (HpLG) has more enhanced cytotoxic and apoptotic tendencies than chronic H. pylori gastritis. A 12-year-old girl with postprandial epigastric pain was diagnosed HpLG on endoscopic biopsy. After the 1st eradication therapy, H. pylori bacilli were still found, and urea breathing test was positive. Although the endoscopic finding was partially improved, clinical symptoms and histologic finding were persisted. We could achieve the improvement of clinical symptoms and disappearance of IELs after the 2nd eradication. The discordant of histopathologic and endoscopic improvement occurred after the 1st eradication therapy of HpLG. Therefore the clinical and histopathologic evaluation should be considered as well as endoscopic findings.
Lymphocytic gastritis (LG), the first described in 1986, is a rare histopathologic subtype of chronic gastritis [1,2]. It is found less than about 1.5% in gastric mucosa biopsy specimens of chronic gastritis [1,2,3]. The histopathologic finding of LG is characterized as dense proliferation of intraepithelial lymphocytes (IELs) more than 25 lymphocytes per 100 epithelial cells [1,3,4].
The pathogenesis is unclear, but celiac disease and Helicobacter pylori infection are known as major causes of LG [1,3,4]. The most common endoscopic finding of LG is varioliform gastritis characterized by severe mucosal nodularity, hypertrophic rugae and erosion predominating on the gastric body [2]. However, it could often be found as normal or non-specific gastric mucosa [3,4,5].
Compared to the adult LG, the pediatric LG is rarely reported. Furthermore, the majority of pediatric LG is associated with celiac disease because 42.1% of children with celiac disease have LG [1]. H. pylori associated LG (HpLG) is less than celiac disease associated LG in children. HpLG are rarely found in age under the 2nd decade [1,6,7,8].
In LG, the proliferation of IELs is the most pathognomonic finding which can distinguish LG from the other chronic gastritis. IELs are functionally and phenotypically distinct from peripheral lymphocytes. IELs are thought to play important roles including mucosal immunity, cell turnover, and apoptosis in mucosa epithelial layer. And the majority of IELs is CD3+/CD8+ T lymphocyte as known as cytotoxic T cell [9]. In addition, cytotoxic and apoptotic tendencies are more enhanced in HpLG than general chronic H. pylori gastritis (CHpG) [3,9,10]. Therefore, clinicians should consider these differences of LG and CHpG in establishing diagnosis and treatment.
The authors report a case of HpLG in 12-year-old girl who was treated with eradication therapies twice due to failure of the 1st eradication therapy. In this case, the authors could sequentially observe and compare the relationship between serial endoscopic, histologic findings, and clinical symptoms during the 1st and the 2nd eradication therapies.
A 12-year-old female presented with nausea, vomiting, and recently aggravating epigastric pain for 5days. She had been suffered intermittent epigastric discomfort for more than 12 months. Her abdominal pain was mild to moderate, intermittent, and postprandial aggravating pain without radiating. Including previous medication history, other past medical or familial histories were not contributory. Her height was 149.1 cm (the 25 to 50 percentile), and weight was 34 kg (the 10 to 25 percentile). Mild epigastric tenderness was noted on physical examination.
The complete blood cell count data were as follows: white blood cell 5,100/mm3, hemoglobin 15.0 g/dL, hematocrit 43.0%. C-reactive protein, serum electrolytes, and the enzymes of liver and pancreas were unremarkable. The result of qualitative anti-H. pylori IgG was positive and the carbon urea breath test (UBT) was also positive as 516 CPM. The plain abdominal radiogram was normal.
The patient underwent endoscopy based on out-patient clinic. Her endoscopic finding was diffuse varioliform gastritis as moderate nodular mucosal change on whole gastric region (Fig. 1A and Fig. 1B). The varioliform was more prominent on gastric body and angle than antrum. The gastric mucosal change was mild on antrum, but moderate on body and angle. Biopsy was done on five sites as guideline of 'the update Sydney system'. Atrophic mucosal change was not found on all biopsy samples. According to 'the update Sydney system', gastritis was grade 2 and stage 0. The histopathologic findings from gastric angle revealed the dense proliferation of IELs (more than 50 per 100 epithelial cells) without the gland destruction (Fig. 2A). And lymphoepithelial lesions as the characteristic histologic finding in of mucosa-associated lymphoid tissue (MALT) lymphoma were not found in lamina propria. On immunohistochemistry, these cells were mostly positive for CD3 (Fig. 2B) and CD8 (Fig. 2C), but negative for CD4 or CD20.
The eradication therapy was done with lansoprazole (15 mg/dose, twice per day), amoxicillin (25 mg/kg/dose, twice per day), and clarithromycin (500 mg/dose, twice per day), for 7 days. Follow-up UBT was still positive (548 CPM) 8 weeks after the end of the 1st eradication. And the abdominal symptoms still persisted as intermittent vomiting, nausea, and abdominal discomfort during follow-up of 12 weeks after the end of the 1st eradication. The follow-up endoscopy was done. Although mild nodularity was remained on angle, varioliform gastritis was partially improved to grade 1 on 'the update Sydney system' (Fig. 1C). On histologic finding with Warthin-Starry silver stain, H. pylori like bacilli were still identified. Although the number of the IELs was partially decreased, 25-50 IELs per 100 epithelial cells were still counted (Fig. 2D) and most of them were positive for CD8 on immunohistochemistry (Fig. 2E).
Because of persistent clinical symptoms, the 2nd eradication was performed for 7 days with bismuth subcitrate (300 mg/dose, twice per day), metronidazole (10 mg/kg/dose, twice per day) and amoxicillin (25 mg/kg/dose, twice per day), although the endoscopic findings improved partially. After the 2nd eradication, clinical symptoms were resolved. The result of follow-up UBT converted to negative as 8 CPM 8 weeks after the end of the 2nd eradication. Gastric mucosa was normal on follow-up endoscopy 5 months after the end of the 2nd eradication (Fig. 1D). H. pylori like bacilli were not found and the density of IELs was normalized (Fig. 2F).
Although lymphocyte proliferation of gastric mucosa is commonly noted in both gastritis subtypes, there are some differences between HpLG and CHpG in clinical and histopathologic aspects.
At the first, the lesion of CD3+ lymphocytic proliferation is intraepithelial layer in HpLG, whereas lamina propria in CHpG. Furthermore, there are some differences of proliferating lymphocyte subsets. In CHpG, the increase range of CD4+ T lymphocytes is generally larger than that of CD8+ T lymphocytes. Therefore, the CD4+/CD8+ lymphocyte ratio is increased in CHpG compared to normal or HpLG [11,12]. On the other hand, most of proliferating lymphocytes in HpLG are CD3+/CD8+ IELs (about 80% [3]) which is cytotoxic T lymphocyte [3,9,10] and they proliferate in intraepithelial layer, not in lamina propria [10]. Furthermore, CD4+/CD25high regulatory T cells, which regulate immune responses, are enriched in gastric mucosa of CHpG [13] but they are not observed in intraepithelial layer of HpLG.
Moreover, it is known that HpLG has more enhanced cytotoxic and apoptotic tendencies than CHpG. A few evidences indicate that the cytotoxicity enhancing molecules such as T cell-restricted intracellular antigen-1 and granzyme B are more increased in HpLG than CHpG [9,10]. The cellular apoptosis is more active in HpLG than CHpG on apoptosis assay with in situ labeling of nuclear DNA fragment' technique [9].
The prognosis of LG is dependent on the elimination or control of the underlying causes such as celiac disease or H. pylori infection. If underlying causes are removed, the clinical symptoms and histopathologic findings are reversibly normalized except apoptotic mucosal change [3]. There are reliable evidences that each of H. pylori infection and LG can be an independent risk factor of gastric lymphoma such as MALT lymphoma [14,15,16].
Because pathogenesis of HpLG differs from that of CHpG, it is necessary that the clinical and therapeutic strategies of HpLG should be considered differently. According to a study of H. pylori eradication in LG, the eradication with triple regimens was more effective on clinical and histologic remission than proton pump inhibitor single therapy [17]. It is known that the eradication of H. pylori can lead to diminish IELs' number [3,18], disease activity and gastritis on endoscopic finding [18] with long-standing resolution of LG [17].
Although endoscopic findings achieved partial improvement in this case, the 1st eradication was not satisfactory as symptom and IELs' proliferation persisted. The reasons of this discordant are thought as two as below. The first is that the improvement of varioliform gastritis doesn't mean as the improvement of LG, because LG doesn't always appear as varioliform gastritis [3,4,5]. Even though the most of HpLG shows varioliform gastritis, some cases show non-varioliform or nonspecific findings on endoscopic examination [19]. The second is that gastric mucosa could have been partially improved by proton pump inhibitor in spite of unsuccessful eradication as previously reported [17].
In conclusion, HpLG has more enhanced cytotoxic and apoptotic tendencies than CHpG. Therefore, active consideration is needed for diagnosis and eradication therapy in the HpLG patients. In the step of diagnosis, clinicians should consider comprehensively the clinical and histologic evaluation as well as endoscopic findings because the discordant between histology and endoscopic finding may appear such as this case. More case reports or further studies regarding pediatric HpLG are needed, because the pathophysiology of HpLG is differ from that of CHpG.
Figures and Tables
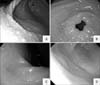 | Fig. 1Endoscopic findings. (A) and (B) varioliform gastritis was shown before beginning of the 1st eradication treatment. It was more severe on gastric body and angle than antrum. (C) After the 1st eradication, varioliform gastritis had improved considerably but mild nodularity was remained on angle. (D) After the 2nd eradication, gastric mucosa became normalized. |
 | Fig. 2Histologic and immunohistochemistry findings. (A) More than 50 intraepithelial lymphocytes (IELs) per 100 epithelial cells were present in the intraepithelial layer (H&E, ×400). On immunohistochemical staining, (B) IELs were mostly positive for CD3 (×400) and (C) CD8 (×400). (D) After the 1st eradication, the number of IELs was decreased but still counted as 25-50 IELs per 100 epithelial cells (H&E, ×400). (E) Majority of IELs were positive for CD8 immunohistochemical staining (×200). (F) After the 2nd eradication, gastric mucosa was normalized (H&E, ×200). |
References
1. Prasad KK, Thapa BR, Lal S, Sharma AK, Nain CK, Singh K. Lymphocytic gastritis and celiac disease in indian children: evidence of a positive relation. J Pediatr Gastroenterol Nutr. 2008; 47:568–572.

2. Haot J, Hamichi L, Wallez L, Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988; 29:1258–1264.

3. Hayat M, Arora DS, Dixon MF, Clark B, O'Mahony S. Effects of Helicobacter pylori eradication on the natural history of lymphocytic gastritis. Gut. 1999; 45:495–498.

4. Wu TT, Hamilton SR. Lymphocytic gastritis: association with etiology and topology. Am J Surg Pathol. 1999; 23:153–158.

5. Broide E, Sandbank J, Scapa E, Kimchi NA, Shapiro M, Lerner A. The immunohistochemistry profile of lymphocytic gastritis in celiac disease and Helicobacter pylori infection: interplay between infection and inflammation. Mediators Inflamm. 2007; 2007:81838.
6. Bhatti TR, Jatla M, Verma R, Bierly P, Russo PA, Ruchelli ED. Lymphocytic gastritis in pediatric celiac disease. Pediatr Dev Pathol. 2011; 14:280–283.

7. De Giacomo C, Gianatti A, Negrini R, Perotti P, Bawa P, Maggiore G, et al. Lymphocytic gastritis: a positive relationship with celiac disease. J Pediatr. 1994; 124:57–62.

8. Luzza F, Mancuso M, Imeneo M, Mesuraca L, Contaldo A, Giancotti L, et al. Helicobacter pylori infection in children with celiac disease: prevalence and clinicopathologic features. J Pediatr Gastroenterol Nutr. 1999; 28:143–146.

9. Han SH, Joo M, Kim KM. High proportion of granzyme B+ intraepithelial lymphocytes contributes to epithelial apoptosis in Helicobacter pylori-associated lymphocytic gastritis. Helicobacter. 2013; 18:290–298.

10. Oberhuber G, Bodingbauer M, Mosberger I, Stolte M, Vogelsang H. High proportion of granzyme B-positive (activated) intraepithelial and lamina propria lymphocytes in lymphocytic gastritis. Am J Surg Pathol. 1998; 22:450–458.

11. Quiding-Järbrink M, Lundin BS, Lönroth H, Svennerholm AM. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin Exp Immunol. 2001; 123:81–87.

12. Lu AP, Zhang SS, Zha QL, Ju DH, Wu H, Jia HW, et al. Correlation between CD4, CD8 cell infiltration in gastric mucosa, Helicobacter pylori infection and symptoms in patients with chronic gastritis. World J Gastroenterol. 2005; 11:2486–2490.

13. Wu YY, Chen JH, Kao JT, Liu KC, Lai CH, Wang YM, et al. Expression of CD25(high) regulatory T cells and PD-1 in gastric infiltrating CD4(+) T lymphocytes in patients with Helicobacter pylori infection. Clin Vaccine Immunol. 2011; 18:1198–1201.

14. Miettinen A, Karttunen TJ, Alavaikko M. Lymphocytic gastritis and Helicobacter pylori infection in gastric lymphoma. Gut. 1995; 37:471–476.

15. Nga ME, Tan SH, Teh M, Koay ES, Chong SM, Putti TC, et al. Lymphocytic gastritis-like T cell lymphoma: molecular evidence of an unusual recurrence. J Clin Pathol. 2004; 57:1222–1224.

16. Song DE, Kim JS, Huh JR, Choi J, Jang SJ, Yu E. Lymphocytic gastritis in Helicobacter pylori-positive gastric MALT lymphoma--report of two cases. Korean J Gastroenterol. 2005; 45:354–360.
17. Madisch A, Miehlke S, Neuber F, Morgner A, Kuhlisch E, Rappel S, et al. Healing of lymphocytic gastritis after Helicobacter pylori eradication therapy--a randomized, double-blind, placebo-controlled multicentre trial. Aliment Pharmacol Ther. 2006; 23:473–479.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download