Abstract
Omega (ω)-3 polyunsaturated fatty acids appear to be effective in preventing and treating parenteral nutrition-associated liver disease, and several mechanisms were proposed for this observation. An 8-week-old male infant with cholestasis and acholic stool was diagnosed non-syndromic intrahepatic interlobular bile duct paucity by open-wedge liver biopsy. Initially he was treated with usual supportive medical therapy, including ursodeoxycholic acid. However, the clinical status and laboratory tests did not improve. Omega (ω)-3 polyunsaturated fatty acids (initially intravenous administration and oral administration later), were started and his liver function, including aminotransferase level and bilirubin levels normalized, and the ivory stool color turned green. We report the possible effectiveness of ω-3 polyunsaturated fatty acids as a potent choleretic agent for non-syndromic intrahepatic interlobular bile duct paucity, a very rare structural pediatric hepatic disease.
Fish oil-based lipid emulsions that are mainly composed of omega (ω)-3 polyunsaturated fatty acids (PUFAs) appear to be effective in preventing and treating parenteral nutrition-associated liver disease [1,2,3,4]. Here, we report the possible effectiveness of an ω-3 PUFA mixture as a potent choleretic agent for non-syndromic intrahepatic interlobular bile duct paucity, a very rare structural pediatric hepatic disease.
An 8-week-old male infant was admitted for the evaluation of jaundice. His stool had been acholic since birth. he was on formula feeding, and the physical examination, perinatal and family histories were not informative. A blood test revealed the following: hemoglobin, 11.6 g/dL; aspartate aminotransferase (AST)/alanine aminotransferase (ALT), 99/34 IU/L; total bilirubin (TB)/direct bilirubin (DB), 12.5/8.7 mg/dL; serum alkaline phosphatase (ALP), 886 IU/L; gamma glutamyl transferase (GGT), 197 IU/L; bile acid, >150 µmol/L; and total cholesterol (TC), 181 mg/dL. Viral markers (HAV, HBV, HCV, Epstein-Barr virus, toxoplasma, rubella, cytomegalovirus, herpes virus, and human immunodeficiency virus), a syphilis test, tandem mass neonatal metabolic screening tests (45 items), an infantogram, and echocardiography were normal. On hospital day 2 (HD#2), magnetic resonance cholangiopancreatography (MRCP) revealed an abrupt cut-off of the first-order right proximal intrahepatic duct and a change in the parenchymal signal intensity in the right lobe of the liver. The administration of ursodeoxycholic acid (UDCA) was initiated on HD#4, at 20 mg/kg/day, and continued throughout the patient's clinical course. Upon hepatobiliary scintigraphy, biliary excretion into the bowel was not noted until after a 24-hour delay. An intraoperative cholangiogram (HD#7) showed an abrupt cut-off of the first order right proximal intrahepatic duct. An open-wedge liver biopsy showed interlobular bile duct paucity with bile ductular proliferation and, severe cholestasis with feathery degeneration (Fig. 1). The administration of a 20% SMOF® lipid emulsion (Fresenius Kabi Austria GmbH, Graz, Austria; α-linolenic acid 0.6 g, eicosapentaenoic acid [EPA] 0.6 g, and docosahexaenoic acid [DHA] 0.1 g/200 g fat/L) was initiated at 2 mL/hr (2 g/kg/d) on HD#18 (AST/ALT, 148/16 IU/L; TB/DB, 10.7/8.2 mg/dL; TC, 77 mg/dL; triglyceride [TG], 110 mg/dL). After treatment for 5 days (HD#23), the stool turned a green color (AST/ALT, 84/36 IU/L; TB/DB, 6.0/4.3 mg/dL; TC, 156 mg/dL). The SMOF® lipid administration was tapered off gradually to 0.5 mL/hr (0.5 g/kg/d) from HD#27 through HD#29. The stool became acholic again (AST/ALT, 157/59 IU/L; TB/DB, 8.2/5.8 mg/dL; TC, 189 mg/dL; TG, 103 mg/dL), and the SMOF® lipid administration was increased to 2.0 mL/hr (2 g/kg/d). After another 4 days, the color of the stool turned green again (AST/ALT, 95/38 IU/L; TB/DB, 6.5/5.2 mg/dL; TC, 222 mg/dL; TG, 119 mg/dL). To prepare for long-term management, an oral ω-3 PUFA agent, Omacor® (ω-3 acid ethyl ester 90; Kuhnil Pharmacy Co., Cheonan, Korea; 840 mg, comprising EPA-ethyl ester [460 mg] and DHA-ethyl ester [380 mg] per 1 g capsule), was initiated on HD#36 and was increased gradually through HD#47 to 3.0 g/day, divided into 6 doses per day. Simultaneously, SMOF® lipid administration was tapered off gradually and was terminated on HD#49. Follow-up hepatobiliary scintigraphy (HD#49) showed normal biliary excretion into the small intestine. Follow-up MRCP (HD#55) showed good visualization of the first order intra hepatic ducts in both lobes of the liver, and the parenchymal signal intensity in the right lobe of the liver was normal (AST/ALT, 91/36 IU/L; TB/DB, 2.9/2.1 mg/dL; TC, 135 mg/dL; TG, 77 mg/dL). He was discharged with UDCA, multivitamines and Omacor®. The stool color was green throughout the patient's clinical follow-up, about 1 year after discharge, with normal liver function test results (AST/ALT, 33/19 IU/L; TB/DB, 0.2/0.1 mg/dL; TC, 192 mg/dL) (Fig. 2).
Recent studies have reported that fish oil-based lipid emulsions appear to be effective in preventing and treating parenteral nutrition-associated liver disease. We referred the therapeutic dose of ω-3 PUFAs in previous studies for this patient [1,2,3,4]. The proposed mechanisms for this phenomenon include altered intracellular lipid peroxidation [5], enhanced production of less inflammatory eicosanoids [6,7,8], and enhanced bile acid excretion [9,10]. However, another study showed that hepatic fibrosis persists and progresses, despite the biochemical improvements, in children treated with intravenous fish oil emulsion [11]. In the present case, we demonstrate the possibility of using an ω-3 PUFA mixture as a potent choleretic agent in a very rare structural pediatric hepatic disease: non-syndromic intrahepatic interlobular bile duct paucity. To the best of our knowledge, this report is the first to show such a case and treatment. In the present case, the administration of ω-3 PUFAs resulted in better outcomes than the usual choleretic agent, UDCA. The exact mechanism responsible for this phenomenon is unclear. However, we do not think that phytosterols played any role in this case. In contrast, we think that the ω-3 PUFAs might have enhanced bile flow and improved the clinical state of the patient. In one study, D-site binding protein (DBP) and liver X receptor-alpha which are known to increase transcriptional level of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme for bile acid production and cholesterol excretion, were increased in mice on a omega-3 fatty acid-rich diet [9]. Based on our experience, we cautiously suggest that the administration of ω-3 PUFAs might be applied in the management of various other pediatric liver diseases that complicate cholestasis, including neonatal hepatitis, the syndromic-type of intrahepatic bile duct paucity, and extrahepatic biliary atresia with Kasai operation.
In most previous studies, the administration of ω-3 PUFAs was performed via the parenteral route [1,3,4]. In several other studies, ω-3 PUFAs were administered enterally. In infants with short bowel syndrome, improvements in cholestasis were observed when soybean lipid emulsion was eliminated from parenteral nutrition, which was then supplemented with enteral fish oil [2]. Another study showed that in mice that underwent bile duct ligation, a Menhaden diet (ω-3 diet) resulted in a trend toward biochemical protection and marked reductions in necrosis and inflammation [12]. However, a recent study showed that dietary fish ω-3 fatty acids supplementation tended to increase liver fibrosis and compromised hepatic function in bile duct-ligated rats [13]. In the present case, the purified oral ω-3 PUFA agent, Omacor®, was used as an alternative to parenteral ω-3 PUFAs, and this treatment replaced the parenteral formula successfully. Further investigations are warranted on the role of ω-3 PUFAs (as a formula for parenteral administration or for oral administration) in pediatric cholestatic liver disease, structural or functional.
Figures and Tables
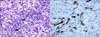 | Fig. 1Liver biopsy showed intrahepatic interlobuar bile duct paucity. (A) On pathological examination, the portal tract showed arteries (thick arrows). However, no corresponding bile ducts of similar caliber was found (H&E, ×400). (B) Immunostaining for CK19 showed extensive bile ductular proliferation (×400). The portal tract showed arteries (thick arrow), but no corresponding bile ducts of a similar caliber was found. |
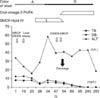 | Fig. 2Schematic of the clinical course of the patient. The patient showed biochemical improvement, and the stool color became green after the treatment with omega-3(ω) polyunsaturated fatty acid. A: acholic stool, G: greenish stool, PUFA: polyunsaturated fatty acid, MRCP: magnetic resonance cholangiopancreatography, DISIDA: diisopropyl iminodiacetic acid scan, TB: total bilirubin, DB: direct bilirubin, ALT: alanine aminotransferase. |
References
1. de Meijer VE, Gura KM, Le HD, Meisel JA, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009; 33:541–547.

2. Rollins MD, Scaife ER, Jackson WD, Meyers RL, Mulroy CW, Book LS. Elimination of soybean lipid emulsion in parenteral nutrition and supplementation with enteral fish oil improve cholestasis in infants with short bowel syndrome. Nutr Clin Pract. 2010; 25:199–204.

3. Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008; 121:e678–e686.

4. Alwayn IP, Gura K, Nosé V, Zausche B, Javid P, Garza J, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005; 57:445–452.

5. Clarke SD, Jump DB. Dietary polyunsaturated fatty acid regulation of gene transcription. Annu Rev Nutr. 1994; 14:83–98.

6. Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003; 100:1751–1756.

8. Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002; 196:1025–1037.
9. Bérard AM, Dumon MF, Darmon M. Dietary fish oil up-regulates cholesterol 7alpha-hydroxylase mRNA in mouse liver leading to an increase in bile acid and cholesterol excretion. FEBS Lett. 2004; 559:125–128.

10. Ramaprasad TR, Srinivasan K, Baskaran V, Sambaiah K, Lokesh BR. Spray-dried milk supplemented with alpha-linolenic acid or eicosapentaenoic acid and docosahexaenoic acid decreases HMG Co A reductase activity and increases biliary secretion of lipids in rats. Steroids. 2006; 71:409–415.

11. Mercer DF, Hobson BD, Fischer RT, Talmon GA, Perry DA, Gerhardt BK, et al. Hepatic fibrosis persists and progresses despite biochemical improvement in children treated with intravenous fish oil emulsion. J Pediatr Gastroenterol Nutr. 2013; 56:364–369.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download