Abstract
Purpose
Iron deficiency remains a very common nutritional problem despite the improvement in nutrition and increased understanding of methods for its prevention. Thus, we try to create a new method for screening iron nutrition through infant nutrition history.
Methods
Among the children who visited Inha University Hospital from March 2006 to July 2012, 181 children with iron deficiency anemia (IDA) and 52 children without IDA ranging from 6 to 36 months of age were reviewed in this study. We used the age when they began to wean food, the type of sort weaning foods, the time required for successful weaning, iron content in weaning foods, and the duration of breastfeeding for scoring infant nutrition history based on a questionnaire.
Results
The mean score of the IDA group was 7.8±2.6 points, which was significantly higher than that of the control group (5.6±2.1) (p=0.000). If we set up the cutoff value at 6 points, this screening has 86.8% sensitivity and 36% specificity. In addition, as the IDA score increased, there was a falling trend of hemoglobin.
The incidence of iron deficiency anemia (IDA) has significantly decreased over time due to breastfeeding, improvement of overall nutritional status, and use of iron-fortified baby food. However, IDA is still observed in infants not only in developing countries, but also in developed countries [1-4]. In Korea, the prevalence rates of iron deficiency and IDA in infants aged 1 and 2 years have been reported as 31.6% and 5.3%, respectively [2]. According to the National Health Insurance Corporation that analyzed data from 2002 to 2008, the number of IDA patients aged less than 1 year increased by 7.2-fold [5]. Furthermore, Yoon [6] used a capillary blood sampling method and reported that the prevalence rates of anemia with hemoglobin (Hb) level less than 11 g/dL in 6-month- and 18-month-old infants were 32.3% and 28.4%, respectively. The prevalence rates of iron deficiency would be even higher than the abovementioned values if the iron status included testing of serum ferritin, iron, and total iron binding capacity, as depletion or iron deficiency occurs prior to the appearance of anemia.
Further, patients with IDA may not be identified because blood sampling from infants is difficult and hence, avoided. Similarly, according to the investigations performed in our clinic, only 18.6% of infants who were diagnosed with IDA visited our clinic because anemia was suspected due to symptoms such as pallor, whereas most IDA cases were identified after performing blood sampling from infants who visited the clinic for other reasons such as respiratory diseases, viral infections, gastrointestinal infections, or vaccinations [7]. The possibility of these infants not being treated for anemia or IDA would be higher if they did not visit the hospital because of other symptoms or diseases.
In addition to the symptoms caused solely by anemia, IDA is also known for its harmful effects on neurological, motor, and behavioral development in infants. Infants with IDA are prone to infections due to suppressed immune function and show loss of appetite, slower growth [8], and poor motor and mental development [9,10]. Children who have IDA in their infancy might have permanent risk of developmental disability, regardless of continuous iron supplementation [11,12]. Brain damage due to iron deficiency in infancy is concerning because most brain development occurs during infancy.
Prevention may be the best way to avoid the negative consequences of iron deficiency on health. However, practically, it is not possible to determine the iron status of every infant by blood sampling. Hence, the test will be cost effective if it is performed on selected infants who are assumed to have risks of nutritional deficiency. Thus, to assist in the selection of infants who should be tested, we aimed at determining if it is possible to predict IDA using data on dietary history or symptoms. Clinically, delayed initiation of weaning, food, nutritional supply solely dependent on breastfeeding for more than 6 months, intake of raw milk before the first birthday, excessive intake of raw milk or juice, and diet composed mainly of foods containing insufficient amount of iron (e.g., thin rice porridge and powered food) were frequently observed in most infants diagnosed with IDA. Despite the various advantages of breastfeeding, one study showed that the iron concentration in breast milk continuously decreases during the first 6 months, decreasing especially rapidly between 1 and 3 months [13]. On the basis of these reports and clinical experience, we believe that selective tests by performing blood sampling on those who have such a dietary history would be an economic and practical alternative.
Thus, we investigated variables that could be used to assess iron status based on the infants' diet and studied the degree of discriminating power for each variable by normalizing the variables.
This study included 254 infants who had participated in the survey conducted among infants aged 6-36 months who had visited Inha University Hospital between March 2006 and July 2012. Infants who had a past history of congenital gastrointestinal diseases that could reduce iron absorption or who were suspected of having anemia due to chronic infection or sepsis caused by long-term fever or who had a surgical history related to congenital gastrointestinal diseases were excluded from the survey. We excluded 21 infants whose body weight at birth was less than 2,000 g as well as 2 infants who were suspected of having other types of hematological malignancies because abnormal levels of white blood cells and platelets were spontaneously observed. Finally, 231 infants were included, and 181 were assigned to the patient group and 50 to the control group. Results of blood tests performed at their visits were retrospectively analyzed. The questionnaire used in this study has been published in the Korean Journal of Pediatrics in 2009 [13].
In the control group, the normal range was defined as 11-16 g/dL for Hb, 33-40% for hematocrit, and 75 fL for mean corpuscular volume (MCV). In the IDA patient group, the normal range was defined <11 g/dL for Hb and <10 ng/mL for serum ferritin or <15% for transferrin saturation.
The IDA scoring table focused on items in the survey completed previously that were assumed to influence the iron status (Table 1). The items selected from the survey were feeding methods for 6 months after birth, age at which weaning food was initiated, infants' overall response to weaning food, and the duration it took for infants to eat various types of weaning food since weaning food was started. The survey included data on the type of weaning food primarily fed, and these were categorized into the following: the homemade group, the commercially purchased group, and the combination group, in which both homemade and commercially purchased weaning foods were used. Formula milk was determined to have higher iron content than breast milk [14]. The homemade weaning food was recategorized into homemade weaning food with high iron content and homemade weaning food with low iron content, according to the main ingredients used in the preparation of weaning food. The ingredients beef, eggs, beans, tofu, fish, seafood, seaweed, nuts, and dairy foods were considered high-iron containing ingredients, and other ingredients including grains, vegetables, fruits, potatoes, and sweet potatoes were considered as low-iron containing ingredients [15,16]. Using medical records, symptoms that were observed at the time of the visit due to IDA, including pallor, pica, and irritability were also retrospectively analyzed.
PASW Statistics 18.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis, and independent samples t-test and Mann-Whitney U test were used for comparison. The data were presented as mean and standard deviation, and the data were determined to be statistically significant when p <0.05. The correlation analysis was done using Spearman's rank correlation coefficient, and the data were determined to be statistically significant when p<0.05.
The demographic data including infants' gender and mothers' age and level of education in the patient and control groups were not statistical significantly different. The average body weight at birth was 3.0 kg in both groups, without any significant difference (p=0.834). The infants' average age was 12.2 months (range, 6-36 months) in the patient group and 11.3 months (range, 6-36 months) in the control group, with the difference being significant (p=0.000). For the 228 infants, reasons for the hospital visit (excluding the ones with unclear records) were fever (45.3%), anemia-related symptoms (23.4%), respiratory symptoms (13.3%), gastrointestinal symptoms (8.6%), convulsion (1.6%), cardiovascular symptoms (0.8%), and others (6.3%) (Table 2). The feeding methods used for infants for 6 months after birth included breastfeeding (87.8%), powdered milk formula feeding (2.8%), and combination feeding in the patient group (9.4%), and breastfeeding (30.0%), powdered milk formula feeding (24.0%), and combination feeding in the control group (46.0%). Hence, there were statistically significant differences in the feeding methods between the 2 groups (p=0.000).
Mean times for initiating weaning food were 6.4±1.7 months in the patient group and 5.9±1.3 months in the control group. The period required for infants to adapt well to weaning food were <1 month for 46.4%, 1-2 months for 23.8%, 3 months for 9.4%, and ≥4 months for 20.4% of the infants in the patient group. The period required for infants to adapt well to weaning food were <1 month for 50.6%, 1-2 months for 28.0%, 3 months for 10.0%, and ≥4 months for 6.0% of the infants in the control group. In the patient group, 78 infants (43.1%) were only breastfed for over 6 months, whereas none of the infants in the control group were only breastfed for over 6 months (Table 3).
The type of weaning food primarily fed to infants in the patient group was homemade only in 75.1%, and commercially purchased or a combination of homemade and commercially purchased weaning food in 24.9% of the patients. The type of weaning food primarily fed to infants in the control group was homemade only in 70.0%, and commercially purchased or combination of homemade and commercially purchased weaning food in 30.0% of the infants (Table 3).
With respect to the ingredients used in the weaning food, only 1 ingredient was used in 27.8% and 25.0% of the homemade food items fed to the infants in the patient and control groups, respectively, and ≥2 ingredients were used in 72.2% and 75.0% of the homemade food items fed to the infants in the patient and control groups, respectively. Among these, foods made using ingredients with high iron content were 51.4% for the patient group and 42.0% for the control group, and foods made using ingredients with low iron contents were 48.6% and 58.0% for the patient and control groups, respectively (Table 3).
The results from IDA scoring, the mean IDA scores were 7.8±2.6 points (range, 2-14) and 5.6±2.1 points (range, 0-10) in the patient and control groups, respectively (Fig. 1). Non-parametric tests were performed, as results from both the patient and control groups did not show normal distribution, and significant differences were found between the 2 groups (p=0.000). Sensitivity of 86.8%, specificity of 36%, positive prediction rate of 81.9%, and negative prediction rate of 18.1% were obtained when the cut-off value was set at 6, according to the measured scores.
The infants were considered to have irritability if they woke up irritated twice or more in the middle of the night and cried with irritation during the day. They were considered to have pica if the infants frequently attempted to consume non-edible matter in cluding paper, dust, and toys. Infants were considered to have pallor if color changes in the lips, conjunctiva, and/or ear conch were observed.
Among 181 of subjects in the patient group, 37 (20.4%) had irritability, 26 (14.4%) had pica, 23 (12.7%) had pallor, 58 (32%) had at least 1 of the symptoms listed above, and 4 (2.2%) had all 3 symptoms. None of the symptoms listed above were observed in the control group. Sensitivity of 32.0% and specificity of 100% were obtained when IDA was diagnosed in patients with 1 or more symptoms among irritability, pica, and pallor.
The comparison of IDA scores and Hb levels for all the surveyed subjects revealed that Hb level, as measured by blood sampling, decreased as the IDA scores (according to the IDA scoring table established in this study) increased (R=0.386, p=0.000) (Fig. 2).
Newborn infants are born with a certain amount of iron, and then ferritin loss occurs around 4-6 months after birth. Rapid growth that takes place during this period may also cause malnutrition; furthermore, iron demand increases during this period. Hence, an adequate supply of iron is required.
Weaning food is not recommended until 6 months after birth in order to prevent atopic dermatitis [17]. The results of the survey performed in this study suggest that nutritional deficiency, especially of iron, may occur in most infants, as 29% of the surveyed infants adapted to the weaning food within 1 month, whereas more than half of the surveyed infants (71%) took more than 1 month to adapt. Moreover, early onset of anemia was reported recently in infants aged less than 6 months [18,19]. As people's perception and knowledge of commercial weaning foods is changing, the use of iron-fortified weaning food has increased; however, IDA is often observed in these cases as well. In addition, the results reveal that the duration required for completely adapting to weaning food has an effect on the onset of iron deficiency because infants are vulnerable to iron deficiency during the period between weaning food initiation and adaptation. When nutrition is provided only by breastfeeding for more than 6 months, iron intake is insufficient. A study reported significantly lower ferritin concentrations in 6-month-old infants who were solely breast fed than in infants who were breastfed with iron supplementation and those who were fed powdered milk formula [20]. It was also reported that the proportion of breastfeeding was 87.4% in infants with IDA, which was significantly higher than that in infants with normal iron status (40.7%) [7]. The possibility of having IDA was higher if weaning food was started later. The thin rice porridge that is given while is low in iron [16]. Moreover, many infants do not eat sufficient quantities of thin rice porridge, resulting in severe iron deficiency. Many clinicians recommend that beef be included as one of the ingredients in weaning food. However, beef, which can improve iron deficiency, is not generally included while initiating weaning food. Furthermore, not all infants eat beef well. In fact, IDA is also observed in infants who were fed iron-fortified weaning food or weaning food containing beef.
We found that more that 75% of infants with IDA were diagnosed with IDA after visiting physicians for other symptoms or diseases; hence, it will be very challenging to identify patients with iron deficiency.
IDA and iron deficiency are known to have irreversible effects on neurological, motor, and behavioral development, regardless of improvement in anemia achieved by adequate iron intake after the diagnosis [21,22]. Considering the harmful effects on the development of infants as anemia progresses, we believe that prevention of IDA by using simple survey methods would be the best practice, allowing early treatment before IDA progression is observed.
The results of this study suggest that a cut-off value should not be set based on only the IDA scores, as IDA scoring had a relatively low (58.6%-86.8%) sensitivity, which should have been higher in order to make the screening test results significant. On the other hand, the results suggest that infants with symptoms including irritability, pica, and pallor should be examined for IDA, as these symptoms have a specificity of 100%. Hence, irritability (3 points), pica (3 points), and pallor (3 points) should be included in the scoring table along with dietary history, although determination of these factors might be subjective.
This study had certain limitations. This study and the survey itself were not analyzed by a double-blinded method, but rather by a retrospective method. Hence, the records for the most common symptoms of IDA, including irritation and pica, were mostly missing. The survey was comprised of pre-established questions en bloc that were answered by parents or caregivers; hence, the results may be influenced by their subjective memory or judgments. Feedback from the experts was not given when the survey was answered. Although in the survey, the parents or caregivers stated the kinds of weaning food fed to the infants, the proportions of the food that the infants actually ate, especially the proportion or frequency of the intake of ingredients with high iron content were not classified further. Finally, we had relatively fewer subjects in the control group than that in the patient group.
However, this study has some strengths. We were able to predict the iron status of infants by performing a simple survey. Hence, it can be easily done in cases when screening tests or full examinations through capillary blood sampling are difficult to perform. In this study, Hb levels were found to decrease as the IDA scores increased. This result suggests that the cut-off value used in screening anemia should be set on the basis of the IDA scoring table, by including more number of subjects. In addition to this, we strongly suggest that tests should be conducted for iron deficiency and IDA in infants showing symptoms of irritation, pica, and pallor, as a sensitivity of 100% was observed for these symptoms.
We recommend that weaning food should be initiated 6 months after birth because of allergic diseases, that is, infants may develop allergy or atopic allergy to the food itself. It is recommended that weaning food should be initiated 6 months after birth for infants who are at risk for atopic dermatitis, as the incidence rate of atopic dermatitis may be higher when weaning food is initiated before 6 months of age [17,23]. However, long-term breastfeeding continuously for more than 6 months and delaying the initiation of weaning food even 6 months after birth may cause IDA in infants who have already suffered from atopic dermatitis or who have relevant risk factors [24]. Although it is well acknowledged that breast feeding for up to 4 months after birth is beneficial for suppressing the onset of atopic dermatitis, whether delaying the initiation of weaning 6 months after the birth suppresses the onset of atopic dermatitis remains unclear [23,25,26]. Guidelines published in 2011 recommend that the initiation of solid food should not be delayed 4.6 months after birth [27]. In this study, when weaning food was initiated after the age of 6 months, more than 30% of infants took 2 or more months to completely adapt to the weaning food.
It is recommended that Hb or hematocrit should be measured 9 months after birth. And, more blood tests are suggested for infants having risk factors for anemia, in order to identify the anemia [28]. Capillary blood sampling is performed due to difficulties in performing venous blood sampling; however, Hb values obtained by capillary blood sampling and venous blood sampling may differ due to dilution of the blood contents by body fluid [29]. There is also the possibility of error in the diagnosis of anemia or IDA, as it does not reveal the morphology of other types of blood corpuscles and important parameters for IDA, including MCV and mean corpuscular Hb. Furthermore, symptoms such as vomiting and diarrhea can sometimes increase the Hb level as well. Therefore, considering the need for precise examination through venous blood sampling, difficulties in the blood sampling itself, and parents' (or caregivers') aversion towards blood sampling, our study results suggest that it is possible to avoid unnecessary blood sampling and missing the identification of infants with anemia if anemia or IDA can be identified using the dietary history of infants.
In conclusion, IDA was observed frequently in infants whose nutrition was supplied only by breastfeeding for more than 6 months, in those who were fed weaning food with low iron content (such as thin rice porridge and powdered food), and in those who took a long time to adapt to the weaning food. Further, the possibility of diagnosing IDA was higher when symptoms such as irritation, pica, and pallor were taken into account. Therefore, iron status should be evaluated for infants with the abovementioned conditions.
Figures and Tables
 | Fig. 1Iron deficiency anemia (IDA) score and comparison of the mean scores of the iron deficiency and control groups. The differences in the IDA scores of the patient group and control groups. The mean score of the IDA group (7.8±2.6) was significantly higher than that of the control group (5.6±2.1) (p=0.000). |
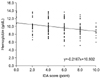 | Fig. 2Correlation between the iron deficiency anemia (IDA) score and hemoglobin (Hb) level. The comparison of the IDA scores and Hb levels for all surveyed subjects revealed that the Hb level decreased as the IDA scores increased (R=0.386, p=0.000). |
References
1. Eden AN, Sandoval C. Iron deficiency in infants and toddlers in the United States. Pediatr Hematol Oncol. 2012; 29:704–709.

2. Yang YJ, Kim SK, Hong YJ, Kim JG, Hyon IY, Hong KS, et al. The prevalence of iron deficiency in preschool children. Korean J Pediatr Hematol Oncol. 1998; 5:14–20.
3. Altuntas N, Beken S, Kulali F, Kazanci E, Unal S, Turan O, et al. Prevalence of iron deficiency at the first age of the infants hospitalized in neonatal period. Transfus Apher Sci. 2012; 47:85–89.

4. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009; 12:444–454.

5. National Health Insurance Corporation. Iron deficiency anemia. accessed 10 Aug 2012. Available from: http://www.nhic.or.kr/portal/site/ida/ida.htm.
6. Yoon JH. Is there the value in screening test for anemia in healthy children with capillary blood sampling? Clin Pediatr Hematol Oncol. 2012; 19:53–56.
7. Choi EH, Jung SH, Jun YH, Lee YJ, Park JY, You JS, et al. Iron deficiency anemia and vitamin D deficiency in breastfed infants. Korean J Pediatr Gastroenterol Nutr. 2010; 13:164–171.

8. Owen GM. Iron nutrition: growth in infancy. Dietary iron: birth to two years. New York: Raven Health Care Communications;1989. p. 103–113.
9. Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993; 341:1–4.

10. Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr. 2010; 91:1684–1690.

11. Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991; 325:687–694.

12. Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000; 105:E51.

13. Chang JH, Cheong WS, Jun YH, Kim SK, Kim HS, Park SK, et al. Weaning food practice in children with iron deficiency anemia. Korean J Pediatr. 2009; 52:159–166.

14. Ahn HS. Pediatrics: Hong Chang Yee. 9th. Seoul: Daehane Textbook Co.;2007. p. 81–85.
15. Martınez-Navarrete N, Camacho M, Martınez-Lahuerta J, Martınez-Monzó J, Fito P. Iron deficiency and iron fortified foods-a review. Food Res Int. 2002; 35:225–231.

16. Kim YN, Na HJ, Kang HJ. Food sources of vitamin and mineral for Korean people (I)-calcium and iron rich foods-. J Korean Home Econ Educ Assoc. 2000; 12:47–64.
17. Kajosaari M, Saarinen UM. Prophylaxis of atopic disease by six months' total solid food elimination. Evaluation of 135 exclusively breast-fed infants of atopic families. Acta Paediatr Scand. 1983; 72:411–414.

18. Kang JU, Jin SH, Choi KD, Jang YT. A study on cow's milk and nursing method in relation to iron deficiency. Korean J Pediatr. 2006; 49:144–149.

19. Sibeko LN, Dhansay MA, Charlton KE, Johns T, Van Stuijvenberg ME, Gray-Donald K. Full-term, peri-urban South African infants under 6 months of age are at risk for early-onset anaemia. Public Health Nutr. 2004; 7:813–820.

20. Noh SJ, Na B, Kim MJ. Iron deficiency and early, low-dose iron supplementation in breast-fed infants. Korean J Pediatr Gastroenterol Nutr. 2008; 11:169–178.

21. Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003; 112:846–854.

23. Schoetzau A, Filipiak-Pittroff B, Franke K, Koletzko S, Von Berg A, Gruebl A, et al. German Infant Nutritional Intervention Study Group. Effect of exclusive breast-feeding and early solid food avoidance on the incidence of atopic dermatitis in high-risk infants at 1 year of age. Pediatr Allergy Immunol. 2002; 13:234–242.

24. Fox AT, Du Toit G, Lang A, Lack G. Food allergy as a risk factor for nutritional rickets. Pediatr Allergy Immunol. 2004; 15:566–569.

25. Stoltzfus RJ, Dreyfuss ML. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington, DC: ILSI Press;1998.
26. Ahn SH, Seo WH, Kim SJ, Hwang SJ, Park HY, Han YS, et al. Risk factors of moderate to severe atopic drmatitis in the first 6 months of life. Pediatr Allergy Respir Dis. 2005; 15:242–249.
27. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011; 31:61–75.

28. Ahn HS. Pediatrics: Hong Chang Yee. 9th. Seoul: Daehane Textbook Co.;2007. p. 105–106.
29. Sari M, de Pee S, Martini E, Herman S, Sugiatmi , Bloem MW, et al. Estimating the prevalence of anaemia: a comparison of three methods. Bull World Health Organ. 2001; 79:506–511.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download