Abstract
Purpose
We investigated the positivity rate and the time period to the positive color change of the urease test in children and adults and assessed the correlation of the urease test to histopathologic findings.
Methods
From 1995 to 2000, endoscopic biopsies of the antrum and body were collected from 811 children and 224 adults and subjected to urease tests and histopathology.
Results
The positivity rate of the urease test was 49.4% for 0-4 years, 48.4% for 5-9 years, 47.3% for 10-15 years, and 62.5% for 20-29 years in the antrum. The positivity rate was 85.1% in 0-4 years, 82.3% in 5-9 years, 74.7% in 10-15 years, and 74.1% in 20-29 years for the body. In the antrum, the highest positivity rate was <1 hour for the group aged 10-29 years and 6-24 hours in the group <10 years old (p<0.0001). In the body, the highest positivity rate was <1 hour in adults and 6-24 hours in children (p<0.0001). The proportions of the positive reactions within 1 hour were similar for the antrum and the body. In the cases of more severe chronic gastritis, active gastritis, and Helicobacter pylori infiltration, a positive urease test reaction occurred more quickly (p<0.0001).
In most cases, Helicobacter pylori infection occurs primarily in childhood [1]. In Korea, H. pylori infiltration, chronic gastritis of more than a moderate degree, and active gastritis have been detected even in children, and an increase in age is gradually accompanied by a greater degree of infection [2].
The diagnostic approaches for H. pylori infection in children do not differ from those used in adults. In general, a culture of the bacteria has been considered to be the gold standard for the diagnosis of H. pylori infection, but the yield of a culture study varies because H. pylori are fastidious bacteria [2]. Other widely used biopsy-based tests include the histologic determination of H. pylori and the urease test. Histopathologic findings for the diagnosis of H. pylori infection are assessed by a specific staining and a specialized pathologist [3]. The urease test is a simple and inexpensive method that requires no special technique to perform and to read the result. Buffered urease tests require at least 1,000 organisms to generate a positive reaction. Therefore, when the histology reveals only 1 or 2 organisms in the entire section, the urease test may be negative [4]. In adults, the rapid urease test is widely used, with a high sensitivity (70-90%) [5]. Data concerning the sensitivity of the urease test in children are controversial [6-8].
In this study, we investigated the positivity rate and the positive timing of the urease test in children and adults using three biopsy specimens from the gastric antrum and body and assessed the correlation of the urease test to histopathologic findings.
After the Institutional Review Board reviewed the research protocols of the present study (GNUHIRB-2012-07-003), 811 children and 224 young adults who underwent endoscopy of the upper digestive tract from 1995 to 2000 were enrolled. In children, the most common indication for upper gastrointestinal endoscopy was upper abdominal pain (75.1%) followed by Henoch- chonlein purpura (H-S purpura) with abdominal pain (8.1%), frequent vomiting (5.3%), heartburn (2.6%) and suspected gastrointestinal bleeding (2.6%). Only 8 patients were diagnosed with hemorrhagic gastritis, gastric ulcers or duodenal ulcers without active bleeding (1 in 0-4 years, 4 in 5-9 years, and 3 in 10-14 years). A total of 80 patients were taking an H2 receptor antagonist at the time of endoscopy (36 in 0-4 years, 34 in 5-9 years and 10 in 10-14 years), and 2 were on steroids due to nephrotic syndrome and adrenogenital syndrome. Most patients were relatively healthy without any underlying disease. A total of 85 patients (10.5%) had underlying diseases such as H-S purpura (n=66), IgA nephropathy (n=10), iron-deficiency anemia (n=4), etc. Many young adults volunteered to undergo endoscopy. About a half of them (n=111, 49.6%) had no gastrointestinal symptoms and the other half had symptoms such as epigastric pain (n=79, 35.3%), dyspepsia (n=26, 11.6%), vomiting (n=3, 1.4%), abdominal distension (n=3, 1.4%), etc. To ensure that the present study only included healthy, young adults, subjects over the age of 30 years were excluded. In this study, the patients with active bleeding were excluded due to the fact that these patients did not undergo biopsy for urease test. All results of the urease tests and the histopathological slides were reviewed. The study populations were stratified into 4 age groups: 0-4 years (n=168), 5-9 years (n=351), 10-14 years (n=292) and 20-29 years (n=224).
Urease tests were performed in the endoscopy room. Briefly, each of the three biopsy specimens from the antrum and the body were incubated in a 2% urea broth (urea 20 g/L, phenol red 0.04 g/L, KH2PO4 0.2 g/L, NaCl 0.5 g/L: pH 6.8), and if a change in color was noted in the following 48 hours, the biopsy was deemed to be urease test positive. The time points at which the positivity occurred were divided into 0-1 hour, 1-6 hours, 6-24 hours, and 24-48 hours. A clinical diagnosis of H. pylori infection was obtained by subjecting the gastric antral and body biopsies to urease tests and histopathology.
Histopathology was performed after the biopsy specimens were fixed in 10% buffered formalin overnight, processed for paraffin embedding, cut into 4-5 µm thick sections, and stained with hematoxylin-eosin (H-E). The histological results were interpreted using the Updated Sydney System. For this, the degrees of lymphocyte (chronicity), neutrophil (activity), and H. pylori infiltration were classified as normal, mild, moderate, or marked.
The data were analyzed using SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA). How the degrees of the urease test and histopathologic findings in the population varied depending on age was evaluated. Statistically significant differences in the positive timing of the urease tests between age groups were determined by using the χ2 test. The relationship in the positive timing of the urease test between the antrum and the body was determined by McNemar's test. p-values of <0.05 were considered to be statistically significant. The inflammatory changes in the gastric antrum and body regions were correlated with the Spearman rank correlation coefficient.
The cases demonstrating a positive color change within 48 hours were 49.4% for 0-4 years, 48.4% for 5-9 years, 47.3% for 10-15 years, and 62.5% for 20-29 years in the antrum. In the body, cases demonstrating a positive color change within 48 hours were 85.1% in 0-4 years, 82.3% in 5-9 years, 74.7% in 10-15 years, and 74.1% in 20-29 years (Table 1). The results of the urease tests in the antrum were significantly different compared with the results of the urease tests in the body, regardless of age (p<0.0001). The positivity rates of the urease test according to age were different for the antrum and the body (p<0.0001).
Cases showing a positive color change within 1 hour and within 6-24 hours in the antrum were 6.0% and 19.0% in 0-4 years, 12.5% and 16.0% in 5-9 years, 25.7% and 7.9% in 10-15 years, and 51.8% and 4.9% in 20-29 years, respectively (Fig. 1). For the body, cases showing a positive color change within 1 hour and within 6-24 hours were 5.4% and 49.4% in 0-4 years, 12.0% and 43.9% in 5-9 years, 22.3% and 27.7% in 10-15 years, and 44.2% and 6.2% in 20-29 years, respectively (Fig. 1). The speed of the positive reaction of the urease test in both the antrum and body was significantly different according to age (p<0.0001). The positivity rate of the urease test in adults primarily occurred within 1 hour in both the antrum and in the body. The proportions of a positive reaction within 1 hour of the urease test were similar between the antrum and the body regardless of age. The positivity rate of the urease test in children primarily occurred within 6-24 hours in the body. The discrepancy in the positivity rates between the antrum and the body in children was composed from the proportion of positive reactions during 6-24 hours in the urease test.
Histopathologic evaluations were performed on 811 children and 224 adults. The proportion of moderate and severe degrees of chronic gastritis in the antrum increased with age, from 10.6% to 53.6%. The proportion of moderate and severe degrees of chronic gastritis in the body also increased with age, from 11.3% to 35.2%. The proportions of active gastritis and H. pylori infiltration in the antrum and body increased with age (Table 2). The degrees of the histopathologic findings in the antrum and body were positively correlated with age (Spearman's R=0.14-0.27, p<0.0001).
When severe chronic gastritis, active gastritis or H. pylori infiltration were found in histopathologic evaluations, the urease tests showed more rapid positive color changes in both the antrum and the body regardless of age compared with those of milder histopathologic grades (p<0.0001). Although bacteria were observed in the histopathologic evaluations, a negative urease test (considered to be a false negative) were observed in 2 (1.2%) in the 0-4 years group, 18 (5.1%) in the 5-9 years group, 11 (3.8%) in the 10-15 years group, and 22 (9.8%) in the 20-29 years group.
In the present study, the positivity rate and the positive timing of the urease test differed according to age. The positivity rate of the urease test using antral biopsy specimens increased with increasing age, and the positivity rate of the urease test using body biopsy specimens decreased with increasing age. The positive urease test had a high concordance with both the density of bacteria and the severity of gastritis [8]. The accuracy of the urease test depends primarily on the H. pylori density in the gastric sample, which is generally lower in children than in adolescents and adults [9]. In this study, the severity of active gastritis and the density of H. pylori in the antrum and body increased with age, and this outcome suggested that the histopathologic findings influence the positivity rate and the positive time in the gastric antrum but not in the gastric body.
In children, a low degree of H. pylori colonization has been noted [9], and the degree of colonization was significantly lower in the body and cardia than in the antrum [10]. These discrepancies could be attributed to sampling errors because of the presence of very low numbers of H. pylori in the tissue samples or because of a patchy distribution of the organism in the stomach's mucosa [11]. In this study, the proportions of the positive color change within 1 hour in the antrum and body were similar in all age groups, and no differences in the speed of the positive reaction in the antrum and the body were observed in the adults. The discrepancy of the urease test between the antrum and the body in children was greater than that in adults, and this outcome resulted from the differences in the proportions of the positive color changes during 6-24 hours. These results suggested that the H. pylori infection persisted from early childhood and that the density of the bacteria increased with age.
In most studies, biopsy specimens were taken from the antrum because this area of the heaviest colonization for H. pylori may be at the lesser curve at the angulus in the prepyloric region [12]. The pooled sensitivity of the urease test increased when samples were obtained from both the antrum and the body [13,14]. In this study, the positivity rate of the urease test in both the antrum and the body was higher comparing with only the antrum. Increasing the number of gastric antral biopsies significantly improves the sensitivity of the rapid urease test and hastens the time required for the test to become positive for the diagnosis [11]. In children aged 1-5 years, the positivity rate of urease test using three biopsy specimens from antrum and body with 48 hour-observation time (86.3%) was higher than using one biopsy specimen from antrum and body with 24 hour-observation period (9.8%) [15]. Comparing the inflammatory changes of the antrum and the body in H. pylori infected children showed a poor correlation with the antrum and the body's density [10]. The degree of the colonization of H. pylori according to the anatomy of stomach (antrum, body, and cardia) differed in several reports [12-14], and the degree of colonization may be related to the differences in either the migration pattern from the antrum to the cardia or to the focal bacteria distribution [10]. In the present study, the histology of the antrum and the body showed a similar severity of gastritis and bacterial density. However, the result of uresase test in the antrum was different in the body. According to the biopsy site in children, the different positive timings of the urease test might be explained by the differences in the density and in the degree of the patchy distribution of bacteria. Debate continues regarding the optimum site and the number of gastric biopsies for the diagnosis of H. pylori.
A false-negative urease test was observed in 3.0% (31/1,037) for the antrum and 1.9% (20/1,037) for the body, and the false-negative urease test results increased with age. These findings are different from other reports that false negatives were higher below the age of 5 years compared with 6 years old and older [16]. False-negative urease tests were associated with the use of acid suppression medication such as proton pump inhibitor (PPI), bleeding, and a very low density of H. pylori [14,15]. The hypochlorhydric subjects had less dense H. pylori colonization, body-predominant colonization and gastritis [17]. The use of acid suppression medication, in particular PPIs, has been shown to reduce H. pylori density and colonization and to suppress the urease activity of the bacterium, thus reducing the accuracy of the test [18]. Another factor related to false-negative urease test was to sampling errors because of a patchy distribution of the organism in the stomach's mucosa [19]. However, the exact reason of false-negative urease tests in this study could not be proved because this study retrospectively reviewed the results of the urease tests and histology only, and no clinical history of volunteers was evaluated.
There are some limitations to the present study. First, we observed the timing of the positive urease test for 48 hours. The buffered urease test received regulatory approval to be read at 24 hours. Although the approved time for reading is different according to the type of urease test, the sensitivity increased over time [20]. A 90% positivity of the CLO-test after 24 hours was reported in 42 infected children, as demonstrated by the Giemsa staining of antral specimens [18]. Second, another limitation of this study was that the histologic diagnosis of H. pylori was based on the H-E stain alone. However, the sensitivity of H-E stains for detecting H. pylori were similar to that of the Giemsa stains [21]. Another limitation is that we were unaware of the volunteers' clinical information, such as medications and underlying diseases. Although they were volunteers, the proportion of moderate and severe degrees of chronic gastritis in the antrum was highest in young adults.
In conclusion, there were significant differences in the positivity rate and the positive timing of urease tests according to age. The discrepancy between the antrum and the body was greater in younger children despite the similarity in all age groups of the proportion of the positive color change within 1 hour in the antrum and body. These results might be related to the low density and patchy distribution of bacteria in children and in the body. In children, the urease test may be more accurate when three or more specimens of both the antrum and body biopsies are used. Further studies concerning the precise time to observe the color change and the exact number of gastric biopsy specimens are needed for urease tests to accurately diagnose H. pylori infection in children.
Figures and Tables
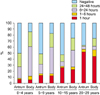 | Fig. 1The positivity rate and positive timing of the urease test both in the antrum and body according to age. The positivity rate of the urease test in the antrum was higher in 20-29 years group comparing with that in other three age groups, and the positivity rate of the urease test in the body decreased with increasing age (p<0.0001). The highest positivity timing was within 1 hour in the 20-29 years group, and within 6-24 hours in children (p<0.0001). The proportions of positive reactions within 1 hour were similar for the antrum and body in all groups. |
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R and D Program for Cancer Control of the Ministry of Health & Welfare of the Republic of Korea (0820050). The biospecimens used in this study were provided by Gyeongsang National University Hospital, which is a member of the National Biobank of Korea that is funded by the Ministry of Health and Welfare.
References
1. Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002. 359:931–935.

2. Ko GH, Park CK, Choi CS, Park HB, Lee JH, Lee HJ, et al. Helicobacter pylori infection and histopathological features of gastric mucosa. Korean J Pathol. 1996. 30:199–209.
3. Ohkusa T, Miwa H, Endo S, Okayasu I, Sato N. Helicobacter pylori is a fragile bacteria when stored at low and ultra-low temperatures. J Gastroenterol Hepatol. 2004. 19:200–204.

4. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996. 20:1161–1181.
5. Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am. 2000. 29:871–878.
6. Graham DY. Helicobacter pylori and the endoscopist: whether to diagnose. Gastrointest Endosc. 1991. 37:577–579.
7. Madani S, Rabah R, Tolia V. Diagnosis of Helicobacter pylori infection from antral biopsies in pediatric patients is urease test that reliable? Dig Dis Sci. 2000. 45:1233–1237.
8. Dondi E, Rapa A, Boldorini R, Fonio P, Zanetta S, Oderda G. High accuracy of noninvasive tests to diagnose Helicobacter pylori infection in very young children. J Pediatr. 2006. 149:817–821.

9. Elitsur Y, Hill I, Lichtman SN, Rosenberg AJ. Prospective comparison of rapid urease tests (PyloriTek, CLO test) for the diagnosis of Helicobacter pylori infection in symptomatic children: a pediatric multicenter study. Am J Gastroenterol. 1998. 93:217–219.

10. Drumm B. Helicobacter pylori in the pediatric patient. Gastroenterol Clin North Am. 1993. 22:169–182.
11. Carelli AP, Patrício FR, Kawakami E. Carditis is related to Helicobacter pylori infection in dyspeptic children and adolescents. Dig Liver Dis. 2007. 39:117–121.

12. Siddique I, Al-Mekhaizeem K, Alateeqi N, Memon A, Hasan F. Diagnosis of Helicobacter pylori: improving the sensitivity of CLOtest by increasing the number of gastric antral biopsies. J Clin Gastroenterol. 2008. 42:356–360.
13. Woo JS, el-Zimaity HM, Genta RM, Yousfi MM, Graham DY. The best gastric site for obtaining a positive rapid ureas test. Helicobacter. 1996. 1:256–259.

14. Tang JH, Liu NJ, Cheng HT, Lee CS, Chu YY, Sung KF, et al. Endoscopic diagnosis of Helicobacter pylori infection by rapid urease test in bleeding peptic ulcers: a prospective case-control study. J Clin Gastroenterol. 2009. 43:133–139.

15. Park JS. Comparison of positive rate of urease tests with different number of biopsy specimens and different biopsy sites according to the age [Unpublished doctoral dissertation]. 2005. Jinju: Gyeongsang National University.
16. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006. 101:848–863.

17. Roma-Giannikou E, Roubani A, Sgouras DN, Panayiotou J, van-Vliet C, Polyzos A, et al. Endoscopic tests for the diagnosis of Helicobacter pylori infection in children: Validation of rapid urease test. Helicobacter. 2010. 15:227–232.

18. Bravo LE, Realpe JL, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroenterol. 1999. 94:2380–2383.

19. El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997. 113:15–24.

20. Oderda G, Dell'Olio D, Morra I, Ansaldi N. Rapid urease test (CLO-Test) for early detection of Campylobacter pylori infection in children. Am J Gastroenterol. 1988. 83:792.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download