Abstract
We present a case of a 7-year-old boy who had cholestasis after trimethoprim-sulfamethoxazole combination therapy. Liver biopsy was performed 36 days after the onset of jaundice because of no evidence of improving cholestasis. Liver histology revealed portal inflammation, bile plug, and biliary stasis around the central vein with the loss of the interlobular bile ducts. Immunohistochemical stains for cytokeratin 7 and 19 were negative. These findings were consistent with those of vanishing bile duct syndrome (VBDS). Chlestasis was progressively improved with dose increment of urosodeoxycholic acid from conventional to high dose. This is the first case report of trimethoprime-sulfamethoxazole associated VBDS in Korean children. The case suggests that differential diagnosis of VBDS should be considered in case of progressive cholestatic hepatitis with elevation of alkaline phosphatase and gamma-glutamyl transpeptidase after or during taking medicine to treat nonhepatobiliary diseases illness.
Vanishing bile duct syndrome (VBDS) is a rare cholestatic liver disease characterized by progressive disappearance of intrahepatic interlobular bile ducts in the absence of underlying liver or biliary tract disease [1,2]. Although VBDS is induced by various conditions, including developmental and genetic abnormalities, immunologic disorders, neoplastic disorders, infectious diseases, and medications, it is mostly associated with drugs [2,3]. More than 30 drugs have been reported as causes of VBDS in adults. However, several cases of drug-induced VBDS have been reported in children: amoxacillin-clavulanic acid-induced VBDS in 3 patients, ibuprofen-induced VBDS in 3 patients, valproic acid-induced VBDS in 1 patient, carbamazepin-induced VBDS in 1 patient, and lamotrigine-induced VBDS in 1 patient [2,4-11]. We present a case of a child who had trimethoprim-sulfamethoxazole (TMP-SMX)-associated VBDS which was resolved after treatment with high-dose ursodeoxycholic acid (UDCA).
A previously healthy 7-year-old boy was hospitalized with jaundice. Two weeks before admission, he had developed mucous diarrhea and mild fever. At that time, he was treated with oral TMP-SMX (trimethoprim 80 mg and sulfamethoxazole 400 mg [Septrin; Samil Pharmaceutical Co., Seoul, Korea], 0.83 tablet twice a day, trimethoprim 8 mg/kg) for 4 days under a presumptive diagnosis of infectious colitis at a private clinic. One day later, he showed icteric sclera, followed by progression of jaundice. After 8 days of the onset of icteric sclera, he was referred to our hospital because jaundice and pruritus became aggravated.
He had no previous history of hepatitis, hepatobiliary diseases, or chronic illness. Family history was nonspecific. Three months before the appearance of jaundice, he received amoxicillin-clavulanic acid for 5 days because of acute tonsillitis at a private clinic.
At admission, he had jaundice and complained of mild epigastric pain and pruritus. On physical examination, his body temperature was 37℃, heart rate 95 beat/min, respiratory rate 22/min, and blood pressure 100/60 mmHg. His growth and development were normal. Although his whole body was icteric, he was not so ill-looking. There was no pharyngotonsillar inflammation and cervical lymph enlargement. Heart and lung sounds on auscultation were normal. The abdomen was soft and flat without tenderness. The liver was palpable 3 finger breath below the right subcostal margin. There was no splenomegaly.
The initial hematologic study showed hemoglobin 11.0 g/dL, white blood cell 4,450/mL (eosinophil 4.3%), platelet 412,000/µL, reticulocyte 1.47%, prothrombin time 10.8 seconds, and partial prothrombin time 27.8 seconds. Liver function tests were as follows: aspartate transaminase (AST)/alanine transaminase (ALT) 231/220 IU/L, total bilirubin (TB)/direct bilirubin (DB) 8.4/7.5 mg/dL, alkaline phosphatase (ALP) 1,028 IU/L, gamma-glutamyl-transpeptidase (GGT) 708 IU/L, and albumin 3.8 g/dL. The total cholesterol level was 490 mg/dL. The Coomb test for direct and indirect was negative. Other serologic tests showed ceruloplasmin 45 mg/dL, immunoglobulin G 851 mg/dL, complement 3/4 each 165/37 mg/dL, antinuclear antibody (-), and antineutrophil cytoplasmic antibody (-). Viral markers for A, B, C, Epstein Barr virus, cytomegalovirus, herpes simplex virus, mycoplasma, and parvovirus were all negative.
Liver ultrasonography and abdominal computed tomography showed normal enhancement patterns of hepatomegaly with no visualization of the common biliary duct and gallbladder. Hepatobiliary scan demonstrated normal liver uptake, but no visualization of the gallbladder and duodenum on 150-minute delayed images (Fig. 1).
Treatment with UDCA (20 mg/kg/day [Ursa; Daewoong Pharmaceutical Co., Seoul, Korea]) was started on hospital day 2. On hospital day 12, liver function test results were similar to those of the admission day showing AST/ALT 103/100 IU/L and TB/DB 8.8/8.0 mg/dL. After discharge from the hospital, he was maintained on UDCA.
Liver biopsy was performed 36 days after the onset of jaundice due to no improvement of cholestasis: TB/DB 8.7/8.6 mg/dL, ALP 709 IU/L, and GGT 548 IU/L. Histologic examination revealed bile plugs and biliary stasis around the central vein with the disappearance of the interlobular bile ducts. Cytokeratins (CKs) 7 and CK 19 were negative by immunohistochemistry. These findings were consistent with those of VBDS (Fig. 2).
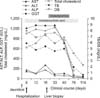
After confirmation of VBDS, UDCA was increased to 30 mg/kg/day. Afterwards, there was improvement in cholestasis. Nine weeks after the onset of jaundice, liver tests at the outpatient clinic showed AST/ALT 77/64 IU/L, TB/DB 1.29/0.66 mg/dL, ALP 815 IU/L, GGT 529 IU/L, and total cholesterol 385 mg/dL. Two weeks later, AST/ALT and total cholesterol normalized. Seven weeks later, ALP and GGT were within the normal range. Clinical progression and treatment course are shown in Fig. 3. Three months after recovery of VBDS, he received amoxicillin-clavulanic acid for 7 days due to acute rhinosinusitis. After that, there was no clinical and laboratory abnormalities associated with VBDS for 1 year. The patient has been followed regularly at the outpatient clinic.
Two to 5 percent of patients admitted to the hospital due to jaundice are related to drug toxicity [12]. Drug-induced cholestasis is presented in either an isolated form or a combined form with some degree of hepatitis, the so-called mixed type of liver injury [3,12]. In the setting of the mixed type of drug-induced cholestasis, a small number of patients may progress to destruction and loss of the intrahepatic bile ducts (ductopenia), resulting in drug-induced VBDS [3,12].
Drug-induced ductopenia is not commonly seen in children. In the literature, a few cases of VBDS induced by amoxacillin-clavulanic acid, ibuprofen, valproic acid, carbamazepine and lamotrigine have been found [2,4-11]. Up to now, TMP-SMX-induced VBDS was found only in adults. This is the first case of TMP-SMX-induced VBDS in children. The important clinical features of adult cases of TMP-SMX-induced cholestasis including VBDS are compared to those of the present case in Table 1.
According to an international consensus report, drug-induced cholestasisis suspected when there is an increase in bilirubin within 5 to 90 days after exposure to a drug and a decrease in cholestasis by 50% within 180 days [13]. The time interval between the first administration of TMP-SMX and the onset of cholestasis was from 1 day to 3 weeks in adult cases, while it was 5 days in the present case (Table 1) [14]. Symptoms associated with drug-induced VBDS are nonspecific, such as fatigue, poor oral intake, abdominal pain, and weight loss. Pruritus due to cholestasis occurs commonly, and gall stone, dyslipidemia, malabsorption, xanthoma of the eyelid, and fat soluble vitamin deficiency are not rare. The present case showed mild abdominal pain, pruritus, and hypercholesterolemia.
Liver chemical tests exhibited mild elevation in transaminase levels, high total and DB concentrations, persistent elevation in ALP and GGT, and hypercholesterolemia [8]. Adults with TMP-SMX-induced cholestasis or VBDS showed an increase in TB from 2.5 to 40.4 mg/dL and normalization of bilirubin concentration achieved from 40 days to 12 months after discontinuation of TMP-SMX (Table 1). In the present case, bilirubin concentration was increased up to 9.9 mg/dL, and biochemical test results normalized 16 weeks after discontinuation of TMP-SMX (Fig. 1).
For the diagnosis of VBDS, extrahepatic biliary obstruction should be excluded in addition to the confirmation of ductopenia and exclusion of other underlying liver diseases by liver biopsy. The liver biopsy of the present case showed bile plugs around the central vein, a widened portal area with loss of interlobular bile ducts, and an increased number of the canal of Hering with CK7 and CK19 being negative by immunohistochemistry. These findings were suggestive of VBDS.
Although it was impossible to prove that TMP-SMX was the cause of VBDS in our patient through TMP-SMX re-challenge, it might be explained by the following findings: (1) no history of hepatobiliary disease, (2) no extrahepatic biliary obstruction or congenital anomaly by radiologic imaging studies, (3) no evidence of immunological factors or infectious causes in blood tests, (4) clear evidence of exposure to TMP-SMX and no clinical or biochemical evidence for amoxicillin-clavulanic acid re-challenge, (5) no inclusion of bile ducts in the portal tract, and negative results in bile duct epithelial cell-specific CK7 and CK19 immunostain.
The pathophysiology of drug-induced VBDS is unclear. Idiosyncratic, metabolic, and immune-mediated mechanisms have been proposed [15]. A drug or its metabolites act as a hapten, which binds to proteins and then produces autoantibodies against CK in the bile duct, resulting in an immune-mediated bile duct injury [15]. Finally, ductopenia appears when T-cell cytotoxicity inducing bile duct injury exceeds the proliferative response [3].
TMP-SMX is commonly used for the treatment of respiratory, gastrointestinal, and genitourinary tract infections. The SMX component of TMP-SMX is related to liver damage [16]. Three types of TMP-SMX-induced liver injury have been described: hepatocelluar injury, mixed hepatocellular cholestatic injury, and bile duct injury with ductopenia or VBDS [16]. The SMX component is oxygenated to SMX hydroxylamine (SMX-NHOH) by the hepatic cytochrome P-450, which can be further oxidized to nitroso-SMX (SMX-NO). These 2 products function as haptens and form hepatoproteins, which induce immune-mediated ductal injury [17].
VBDS is usually treated by discontinuation of the causative drug and initiation of medications that stimulate biliary excretion. The most commonly used drug is UDCA. UDCA is a hydrophilic dihydroxy bile acid that accelerates the transcription of the transporter protein. It stimulates hepatocellular secretion and increases the growth of the hepatocellular surface [3]. It also inhibits hepatocellular apoptosis that is activated by bile acids and protects the hepatocytes by inducing survival signals [3,18]. The standard treatment method for VBDS has not yet been established. Withdrawal of the causative drug and prevention of re-exposure are essential.
The prognosis of drug-induced VBDS is unpredictable and varies according to the degree of bile duct damage. The outcome of drug-induced VBDS may be related to the reproductive ability of small bile ducts. Most of the drug-induced VBDS cases recovered spontaneously like our case. Secondary biliary cirrhosis or liver failure may sometimes occur [2,3].
We described a case of a 7-year-old boy who developed VBDS after administration of TMP-SMX for the treatment of presumptive infectious colitis at a local clinic. Our case suggests that VBDS should be considered as differential diagnosis in patients with progressive cholestasis accompanied by high serum levels of ALP, GGT, and cholesterol after or during medication for the treatment of acute illness.
Figures and Tables
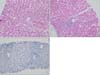 | Fig. 2Histologic findings. (A, B) Bile stasis and bile plugs around the central veins, a widened portal area with loss of interlobular bile ducts, and an increased number of the canal of Hering (H&E, ×200). (C) Negative immunohistochemical staining for cytokeratin 19 (×200). |
References
1. Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol. 1997; 26:Suppl 1. 31–35.

2. Smith LA, Ignacio JR, Winesett MP, Kaiser GC, Lacson AG, Gilbert-Barness E, et al. Vanishing bile duct syndrome: amoxicillin-clavulanic acid associated intra-hepatic cholestasis responsive to ursodeoxycholic acid. J Pediatr Gastroenterol Nutr. 2005; 41:469–473.

4. Gökçe S, Durmaz O, Celtik C, Aydogan A, Güllüoglu M, Sökücü S. Valproic acid-associated vanishing bile duct syndrome. J Child Neurol. 2010; 25:909–911.

5. Chawla A, Kahn E, Yunis EJ, Daum F. Rapidly progressive cholestasis: an unusual reaction to amoxicillin/clavulanic acid therapy in a child. J Pediatr. 2000; 136:121–123.

6. Stricker BH, Van den Broek JW, Keuning J, Eberhardt W, Houben HG, Johnson M, et al. Cholestatic hepatitis due to antibacterial combination of amoxicillin and clavulanic acid (augmentin). Dig Dis Sci. 1989; 34:1576–1580.

7. Srivastava M, Perez-Atayde A, Jonas MM. Drug-associated acute-onset vanishing bile duct and Stevens-Johnson syndromes in a child. Gastroenterology. 1998; 115:743–746.

8. Taghian M, Tran TA, Bresson-Hadni S, Menget A, Felix S, Jacquemin E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr. 2004; 145:273–276.

9. Jang GC, Moon KR, Suh CH. A case of vanishing bile duct syndrome associated with ibuprofen overuse. Korean J Pediatr Gastroenterol Nutr. 2006; 9:262–268.

10. Garcia M, Mhanna MJ, Chung-Park MJ, Davis PH, Srivastava MD. Efficacy of early immunosuppressive therapy in a child with carbamazepine-associated vanishing bile duct and Stevens-Johnson syndromes. Dig Dis Sci. 2002; 47:177–182.
11. Bhayana H, Appasani S, Thapa BR, Das A, Singh K. Lamotrigine-induced vanishing bile duct syndrome in a child. J Pediatr Gastroenterol Nutr. 2012; 55:e147–e148.

12. Geubel AP, Sempoux CL. Drug and toxin-induced bile duct disorders. J Gastroenterol Hepatol. 2000; 15:1232–1238.

13. Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990; 11:272–276.
14. Faria LC, Resende CC, Couto CA, Couto OF, Fonseca LP, Ferrari TC. Severe and prolonged cholestasis caused by trimethoprim-sulfamethoxazole: a case report. Clinics (Sao Paulo). 2009; 64:71–74.

15. Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001; 8:303–315.

16. Abusin S, Johnson S. Sulfamethoxazole/trimethoprim induced liver failure: a case report. Cases J. 2008; 1:44.

17. Yao F, Behling CA, Saab S, Li S, Hart M, Lyche KD. Trimethoprim-sulfamethoxazole-induced vanishing bile duct syndrome. Am J Gastroenterol. 1997; 92:167–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download