Abstract
Congenital esophageal stenosis (CES) can be classified into three types based on the etiology of stenosis: tracheobronchial remnants (TBRs), fibromuscular hypertrophy (FMH), and membranous diaphragm (MD). It is important to make a differential diagnosis because the therapeutic plan for CES is determined by its etiology. Most cases of FMH and MD can be managed with balloon dilatation, whereas cases of TBRs require resection and anastomosis. Thus, the preoperative distinction of TBRs is critical. Recently miniprobe endoscopic ultrasonography (EUS) with a maximum diameter of 2.5 mm has been useful for distinguishing TBRs from FMH in pediatric patients with CES. EUS shows hyperechoic lesions indicating TBR cartilage. Miniprobe EUS is recommended for choosing the correct therapeutic method for CES. We report a case of CES due to TBRs in which a preoperative diagnosis was made in a child using miniprobe EUS without any difficulties.
Congenital esophageal stenosis (CES) is an intrinsic stenosis of the esophagus. It is caused by a congenital malformation of the esophageal wall. The estimated incidence is 1 per 25,000 to 50,000 live births [1]. CES is classified into three histologic entities, including tracheobronchial remnants (TBRs), fibromuscular hypertrophy (FMH), and membranous diaphragm (MD) [2-4]. To distinguish TBRs from FMH and MD is important because TBRs are generally be treated by surgical excision, whereas FMH without a severe stenosis and MD can be treated successfully with balloon dilatation [5].
While esophagography suggests CES and esophagogastroduodenoscopy (EGD) enables the diagnosis of CES in most cases, it is very difficult to make a differential diagnosis of the three histologic entities. Endoscopic ultrasonography (EUS) has proven useful in the diagnosis of CES due to TBRs by showing the presence of cartilage [6]. Conventional radial EUS is limited for use in children or stenotic lesions of the gut because of its large diameter. However, EUS using a mini-catheter probe inserted via the working channel of the endoscopy is a safe and useful tool for evaluating high-grade stenotic lesions [7] and allows for the acquisition of abnormal lesions of gut wall layers even in small children.
Usui et al. [6] reported an experience of applying high-frequency catheter miniprobe EUS to distinguish between FMH and TBRs in children with CES in whom symptoms had not improved by balloon dilatation. We report a case in which miniprobe EUS with a diameter of 2.5 mm was used to diagnose a child with CES due to TBRs. The miniprobe EUS allowed a direct operation without the use of balloon dilatation.
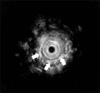
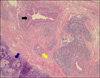
A 12-year-old boy presented with increasing difficulty swallowing solid foods and vomiting. He had vomited frequent since 5 months of age. The cause of the vomiting had not been evaluated. At 5 years of age, he had a history of a foreign body (a bolt) ingestion in the esophagus. The bolt was not spontaneously passed into the stomach, and so it was removed by endoscopy. On admission, he had a body weight of 32 kg (10-25th percentile) and a height of 143 cm (10-25th percentile). On hospital day 2, we performed an upper endoscopy and esophagography. The endoscopy showed a stenotic central lumen at distal 30 cm from the incisor with proximal widening of the lumen (Fig. 1). There was no evidence of mucosal breaks or erosions. The endoscopic probe (Olympus GIF-XQ260, 9 mm diameter) could not be advanced into the stomach. The esophagography showed that there was a circumferential luminal narrowing of the distal esophagus with multiple extraluminal filling of barium and dilatation of the proximal esophagus (Fig. 2). Peristalsis of the esophagus was normal. We suspected that the esophageal lesion was a CES. On hospital day 3, we performed EUS to make a differential diagnosis of TBRs and FMH. EUS was conducted using a high-frequency miniprobe catheter (UMDP20-25R, 20MHz; Olympus Optical, Tokyo, Japan). EUS showed that multiple hyperechogenic spots had spread into the muscle layer of the stenotic lesion (Fig. 3). Thus, the esophageal stenosis was TBRs. A segmental resection and reanastomosis with fundoplication were performed. The pathologic examination showed cartilages, glands and ciliated respiratory epithelium in the muscle layer of the resected esophagus (Fig. 4). There was no leakage at the area of anastomosis after the operation. The patient has been well on follow-up visits.
The cause of CES is not clearly understood. TBRs are the most common, whereas MD is the least common variant [8]. TBRs are caused by a separation abnormality between the respiratory tract and the esophagus [9]. The cause of the anomaly could be intra-uterine stress or anoxia during the first month of pregnancy [8]. TBRs occur in the distal portion of the esophagus, whereas FMH and MD are more common in the middle third portion [10]. CES is usually diagnosed in infancy or childhood. But some cases are diagnosed in adulthood because of the varying degree and location of the stenosis [11,12]. If the stenosis lacks severity, the time of diagnosis might be delayed. Patients with CES typically present with symptoms of vomiting and progressive dysphagia upon ingestion of solid food [11]. Thus, the clinical symptoms and signs of CES do not appear when liquid formula is ingested and may appear at around the weaning period when semisolid and solid foods are introduced into the diet [8]. Jang et al. [4] reported that the median age at diagnosis was 2 years and that the period of delay in diagnosis ranged from 11 months to 84 months. In this case, the patient's symptoms developed at 5 months, and the patient had a history of a foreign body ingestion in the esophagus at 5 years. We think that delaying diagnosis was caused by failure to notice of the esophageal symptoms and the lack of knowledge of this manifestation of CES such as dysphagia, aspiration pneumonia, and failure to thrive.
TBRs and FMH are more frequently associated with esophageal atresia/tracheoesophageal fistula [8]. There were no associated anomalies in the present case.
FMH has a smooth wall with an hourglass configuration and usually develops into a partial obstruction of the lumen. In contrast, TBRs with cartilages often show high-grade stenosis on radiologic fndings [10]. The location and shape of stenosis on esophagography and EGD help us suspect and make a diagnosis of CES. However, it is difficult to make an accurate diagnosis of CES types without pathological information. The TBRs pathologic findings show bronchial accessory glands and tracheal cartilages in a thick smooth muscle layer of esophagus [1]. Segmental hypertrophy of the muscularis and submucosal layers with diffuse fibrosis are seen in patients with FMH [8,13]. CES caused by TBRs tend to require surgical resection, whereas patients with CES caused by FMH and MD can be treated by dilatation or bougienage [5,8]. In many case series, dilatation has played a pivotal role in the treatment of FMH and if the CES is successfully-treated by dilatation, it is considered the FMH type [14]. However, other authors reported that about 70% of patients with CES require surgical treatment [9]. In general, dilatation is initially attempted for FMH and MD, and then if symptoms recur very soon or complications (perforation) develop, surgical intervention should be considered.
Recently, Takamizawa and Usui et al. [5,6] have reported on pediatric patients with CES in whom the TBRs was shown by EUS. Although the usefulness of EUS is well established in adult gastrointestinal tract and pancreaticobiliary diseases, the use of EUS in pediatric patients is uncommon [15]. EUS can show structural abnormalities of the gut wall and aids to distinguishing the origin of subepithelial and submucosal lesions, as well as extraluminal structures [15]. EUS visualizes hyperechoic lesions in the muscle layer due to the cartilage component of TBRs, whereas only the thickness of the muscular layer is visible in FMH. Conventional EUS is not easily performed in young children because of the size of the 12~13 mm endoscopic probe. Furthermore, a stenotic area such as CES and stricture lesions prohibits the passage of a conventional probe [7]. EUS using a miniprobe was performed by inserting the working channel of a flexible conventional EGD [7]. Recently, a case was reported in which a EUS examination was performed by inserting the miniprobe into the working channel of a slim (4.9 mm) endoscope to evaluate an esophageal stenotic area [16]. The EUS miniprobe has a much smaller diameter and a higher frequency compared with conventional EUS [7]. Therefore, the EUS miniprobe is limited and might be useless in cases requiring a full image such as large tumors or abnormal lesions in deep layers [7]. Attila et al. [15] performed EUS in 38 children with a mean age of 13.5 years. The most common indications were pancreaticobiliary lesions. Neoplastic diseases in children are rare, so the use of EUS in pediatric patients is limited compared to that in adults. Submucosal tumors are occasionally found in pediatric patients, also making EUS a possible method to correctly diagnose abnormal lesions on the gut wall layers in pediatric patients [15]. CT or MRI are not useful for detecting TBRs, as they are not visible on radiological imaging [16]. Therefore, miniprobe EUS is a very useful diagnostic tool, particularly in pediatric patients. The distinction between our case and Usui's reported case [6] using a minprobe for diagnosing CES is that we performed EUS with a miniprobe first without dilatation or other interventions. Therefore, we did not encounter complications and were able to make the preoperative diagnosis of CES due to TBRs. Performing a miniprobe EUS should be considered in the initial diagnostic method of CES before dilatation and other interventions are performed.
Figures and Tables
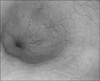 | Fig. 1Esophageal endoscopy shows a stenotic central lumen at 30 cm distal esophagus from the incisor. |
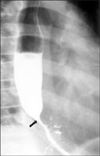 | Fig. 2Esophagography shows a luminal narrowing (arrow) of the distal esophagus with multiple extraluminal filling of barium and dilatation of the proximal esophagus. |
ACKNOWLEDGMENTS
The present research was conducted by the research fund of Dankook University in 2011.
References
1. Saito T, Ise K, Kawahara Y, Yamashita M, Shimizu H, Suzuki H, et al. Congenital esophageal stenosis because of tracheobronchial remnant and treated by circular myectomy: a case report. J Pediatr Surg. 2008. 43:583–585.

2. Maeda K, Hisamatsu C, Hasegawa T, Tanaka H, Okita Y. Circular myectomy for the treatment of congenital esophageal stenosis owing to tracheobronchial remnant. J Pediatr Surg. 2004. 39:1765–1768.

3. Sung GW, Lee SY, Choi YH, Kim KM, Seo JK, Kim IW, et al. Four cases of congenital esophageal stenosis due to tracheobronchial remnants. J Korean Pediatr Soc. 1996. 39:273–279.
4. Jang JY, Ko JS, Park KW, Kim IW, Kim WS, Jang JJ, et al. Congenital esophageal stenosis: with special reference to diagnosis and postoperative complications. J Korean Pediatr Soc. 1999. 42:535–544.
5. Takamizawa S, Tsugawa C, Mouri N, Satoh S, Kanegawa K, Nishijima E, et al. Congenital esophageal stenosis: therapeutic strategy based on etiology. J Pediatr Surg. 2002. 37:197–201.

6. Usui N, Kamata S, Kawahara H, Sawai T, Nakajima K, Soh H, et al. Usefulness of endoscopic ultrasonography in the diagnosis of congenital esophageal stenosis. J Pediatr Surg. 2002. 37:1744–1746.

7. Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008. 12:256–262.

8. Nemolato S, De Hertogh G, Van Eyken P, Faa G, Geboes K. Oesophageal tracheobronchial remnants. Gastroenterol Clin Biol. 2008. 32:779–781.

9. Amae S, Nio M, Kamiyama T, Ishii T, Yoshida S, Hayashi Y, et al. Clinical characteristics and management of congenital esophageal stenosis: a report on 14 cases. J Pediatr Surg. 2003. 38:565–570.

10. Ramesh JC, Ramanujam TM, Jayaram G. Congenital esophageal stenosis: report of three cases, literature review, and a proposed classification. Pediatr Surg Int. 2001. 17:188–192.

11. Oh CH, Levine MS, Katzka DA, Rubesin SE, Pinheiro LW, Amygdalos MA, et al. Congenital esophageal stenosis in adults: clinical and radiographic findings in seven patients. AJR Am J Roentgenol. 2001. 176:1179–1182.
12. Jeong WS, Jeen YT, Chun HJ, Kim DR, Kwon YD, Lee HS, et al. A case of congenital esophageal stenosis due to tracheobronchial remnants in adult. Korean J Gastrointest Endosc. 2003. 26:21–25.
13. Nihoul-Fekete C, De Backer A, Lortat-Jacob S, Pellerin D. Congenital esophageal stenosis: A review of 20 cases. Pediatr Surg Int. 1987. 2:86–92.
14. Jones DW, Kunisaki SM, Teitelbaum DH, Spigland NA, Coran AG. Congenital esophageal stenosis: the differential diagnosis and management. Pediatr Surg Int. 2010. 26:547–551.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download