Abstract
The etiology of peptic ulcer disease in children may be primary, associated with Helicobacter pylori infection, or secondary, relied on underlying disease. Ulcerative lesions by H. pylori are mainly distributed in the duodenal bulb and they are rare below the ampulla of Vater because H. pylori growth is inhibited by bile juice. In this reason, there are only some restrictive reports presented small bowel ulcer associated H. pylori. We found multiple small bowel ulcerative lesions associated with H. pylori in an 11-year-old girl without any systemic disease while performing esophagogastroenteroscopy to the level of the proximal jejunum for differentiating bezoar. The abdominal pain improved after the patient was administered H. pylori eradication therapy. Because a small bowel ulcer associated with H. pylori has rarely been reported, we report it here with literature review.
Peptic ulcer generally refers to gastric and duodenal ulcers. According to etiology, primary peptic ulcers, which are chronic and more often duodenal, are most often associated with Helicobacter pylori infection. In contrast, secondary peptic ulcers, which are more acute and resulted from underlying disease, mainly invade gastric mucosa and affect young children such as neonates and infants [1].
The prevalence of H. pylori infection in children is <10% in developed countries but up to 80% in developing countries [2]. The prevalence of H. pylori infection in children affected by duodenal ulcer is 92% globally [3]. The prevalence of H. pylori infection in Korean school-aged children is about 16.5% [4,5]. Approximately 27% of children with recurrent abdominal pain and 65% of children with duodenal ulcers in Korea suffer from H. pylori infection [4,5]. Therefore, duodenal ulcers are highly associated with H. pylori infection regardless of prevalence. In contrast, nonsteroidal anti-inflammatory drugs (NSAIDs), hypergastrinemia, and Crohn's disease are rare causes of peptic ulcer [6].
Peptic ulcer is caused by an imbalance between mucosal attack factors such as gastric acid and mucosal defense factors such as the epithelial layer, intracellular tight junctions, the mucosal layer, secretion of bicarbonate, and epithelial growth factor and prostaglandin production. Among them, gastric acid is highly related to duodenal ulcers [7].
H. pylori can only survive on gastric mucosa. But, excessive gastric acid secretion caused by increased gastrin secretion by H. pylori induces gastric metaplasia. As a result, colonization of H. pylori and duodenal ulceration may occur. Therefore, ulcerative lesions are often located in the duodenal bulb adjacent to the stomach, thus, an ulcer associated with H. pylori is rare below the ampulla of Vater, except for cases of gastric acid hypersecretion such as Zollinger-Ellison syndrome, as H. pylori growth is inhibited in proportion to bile juice [8].
Ulcers are rare in children, and symptoms are mainly abdominal pain, vomiting, and bleeding. In this article, we report a case of improved symptoms of a small bowel ulcer associated with H. pylori infection in an 11-year-old girl who had undergone a gastrotomy to remove a bezoar and, a few years later, received antisecretory and H. pylori eradication therapy.
An 11-year-old girl presented with a history of stabbing epigastric pain every 10 minutes and nonbilous vomiting more than 10 times a day. She did not have fever or diarrhea.
She had undergone a gastrotomy and removal of an 8 cm trichobezoar 6 years previously. She ate hair on the floor at that time habitually. She had not been administered medication during the past 3 months.
She was 140 cm (25-50th percentile) in height and 34 kg (25-50th percentile) in weight, and her vital signs were: blood pressure, 100/60 mmHg; pulse, 88 beats per minute; respiratory rate, 20 breaths per minute; and temperature, 36.2℃. On admission, she had a clear mental status but seemed acutely ill. Neither conjunctiva were pale, and both sclera were icteric. Respiratory and heart sounds were normal. Abdominal distention was not observed, but bowel sounds were mildly activated. She complained of severe tenderness in the epigastric area with no rebound tenderness. No hepatosplenomegaly or other abdominal mass was detected on abdominal palpation. There was no patchy baldness or abnormalities of the skin or mucosa.
On admission, initial laboratory tests showed hemoglobin, 13.2 g/dL; hematocrit, 38.3%; white blood cell count, 13,260/mm3 (neutrophils 88.5%); platelet count, 272,000/mm3; erythrocyte sedimentation rate, 9 mm/hr; and C-reactive protein, 1.6 mg/L. The biochemical profile consisted of serum protein, 6.7 g/dL; albumin, 4.7 g/dL; total bilirubin, 0.5 mg/dL; direct bilirubin, 0.2 mg/dL; aspartate aminotransferase, 17 IU/L; alanine aminotransferase, 11 IU/L; blood urea nitrogen, 6.8 mg/dL; creatinine, 0.5 mg/dL; and amylase, 55 U/mL. A blood coagulation test showed a prothrombin time-internationalized normal ratio, 1.17; and activated partial thromboplastin time, 27.0 sec. Urine analysis showed specific gravity, 1.025; pH, 6.0; negative for protein, glucose, blood, urobilinogen, bilirubin, and nitrite, but positive for ketone bodies. No white blood cells or red blood cells were observed in the urine.
A simple abdominal X-ray was unremarkable at admission. Abdominal computed tomography (CT) revealed a 4 cm sized low attenuating mass-like lesion in the stomach (Fig. 1). We performed esophagogastroduodenoscopy (EGD) for identifying a recurrence of trichobezoar (Fig. 2). Mucosal erythema was observed in the gastric antrum, but no ulcerative lesions were found in the stomach. A scar, which was considered a gastrotomy suture line, and a duodenal bulb with structural deformity were noted. We suspected transition of the mass below the stomach, so we inserted a longer endoscopic fiber using a CF-Q260AL (Olympus, Tokyo, Japan). We inserted the fiber 60 cm from the incisor without kinking and approached the proximal jejunum. We found multiple longitudinal ulcerative lesions from the duodenal bulb to the proximal jejunum, although there was no trichobezoar or mass causing a mechanical bowel obstruction. We performed a small bowel series after marking the endoscopic approach level using clips (Fig. 3) and found normal contrast transition without obstruction or abnormal findings near the mass. Partial hypertrophy of the bowel mucosal fold was noted, but no abnormality was found in the ileocecal portion. The rapid urease test in the duodenal bulb was negative.
Chronic inflammation with lymphoid follicles was noted on pathological examination of the ulcerative lesion (Fig. 4).
Serum gastrin level and the autoimmune antibody test were performed to determine the cause for the small bowel ulcer. The laboratory findings showed serum gastrin, 57.0 pg/mL; negative anti-neutrophil cytoplasm antibodies and anti-Saccaromyces cerevisiae antibodies; and negative Tb-polymerase chain reaction of the bowel mucosa tissue. The patient received lansoprazole (15 mg/dose, twice a day). On the day of discharge, 4 days after admission, the epigastric pain improved, and she returned to a normal diet. After discharge, she received H. pylori eradication therapy, including lansoprazole (15 mg/dose, twice per day), amoxicillin (50 mg/kg/day in two divided doses), and clarithromycin (30 mg/kg/day in two divided doses) for 2 weeks. She took additional lansoprazole (15 mg/dose, once per day) for 4 weeks, subsequently. Eight weeks after stopping the medication, the urea breath test was negative. The patient has not had any specific symptoms for 18 months.
Before it was determined that peptic ulcers were related to H. pylori, there was only the hypothesis that peptic ulcers resulted from an imbalance between gastric acid secretion and the mucosal defense mechanism. Thus, peptic ulcers are known as primary ulcers. After Warren and Marshall found H. pylori in the gastric mucosa of a patient with gastritis and a duodenal ulcer in 1982 [9], the etiology of peptic ulcer in children and adults was identified as H. pylori infection [10,11]. H. pylori mainly chronically affects children >10 years old and often causes duodenal ulcers without systemic symptoms. The exact role of H. pylori in peptic ulcer has not been clearly elucidated. And it is curious that H. pylori infection precedes symptoms or is related to recurrence after eradication therapy. This is because peptic ulcers have a multifactorial correlation with virulence, quantitative colonization of the bacteria itself, genetic vulnerability of the host, a drug attenuating mucosal defense mechanism, psychological stress, and smoking [12].
In our case, because the CT image showed a mass-like lesion in the stomach, we suspected a mechanical obstruction by a mass and performed EGD. The operative record indicated gastrotomy of the anterior wall of the stomach and evacuation of a trichobezoar. We assumed that the gastrotomy incision included the duodenal bulb, as we detected a longitudinal suture scar in the duodenal bulb during endoscopy. However, no mass was found, so predicting distal transition of the mass, we inserted the endoscopic fiber more forward than usual into the small bowel. Although no mass was found in the small bowel, we found multiple longitudinal ulcerative lesions from the duodenal bulb to the proximal jejunum. We marked the endoscopic approach level using clips and performed a small bowel series immediately to identify transition of the mass and the range of the ulcer. No problem occurred by passing contrast, and mass-like bezoar or structural anomaly of the ileocecal portion were not found. But, mild mucosal fold hypertrophy was suspected in the partial proximal jejunum. A biopsy performed at the margin of the ulcer and a histological examination showed nonspecific chronic inflammation with lymphoid follicles but no gastric metaplasia.
A jejunal ulcer is rare, but some restrictive reports are available about this type of ulcer. Among them, a report showed that jejunal ulcer occurred in an adult with Zollinger-Ellison syndrome, and another report presented an experimental animal model with duodenal and jejunal ulcers caused by hypersecretion of gastric acid as in Zollinger-Ellison syndrome. Aspirin has been associated with jejunal ulcers in some reports [13-15].
In this case, the patient had not taken any medications including NSAIDs for 3 months from the onset of abdominal pain and vomiting. We checked serum gastrin level as a cause for the small bowel ulcer, but it was normal. No evidence of Crohn's disease was found. Gastric acid loading in the duodenum caused by gastric acid hypersecretion should precede colonization of H. pylori in the duodenum. The duodenal bulb is the main site for peptic ulcer, as bile juice secreted into the ampulla of the duodenum is a mucosal defense factor against H. pylori growth. In contrast, increased gastric acid loading in the duodenal bulb can induce weakness of the mucosal defense mechanism, because gastric acid-precipitated bile acid combines with glycine in the form of bile salts. As a result, highly polarized bipolar phospholipids make it easy for gastric acid to infiltrate the mucosa [7].
In our case, the patient may have had a weak mucosal defense mechanism against H. pylori, because a structural deformity of the duodenal bulb caused by gastrotomy can lead to gastric acid overexposure of the mucosa below the duodenal bulb, although bile juice is secreted into the ampulla of Vater.
As the endoscopic fiber is usually introduced up to the second portion of the duodenum during EGD, a diagnosis of peptic ulcer below that level can be restricted. We observed ulcerative lesions below the duodenal bulb, as we performed the endoscopic approach more forward than a conventional procedure due to suspicion of a false mass shown on the CT image.
The patient was administered a proton pump inhibitor (lansoprazole), and the abdominal pain subsided gradually after day 4 of hospitalization. The proton pump inhibitor was maintained for more 4 weeks after eradication of H. pylori. After termination of therapy, symptoms disappeared completely and her parents did not want an endoscopic follow-up, so we confirmed eradication of H. pylori by a negative result on the urease breath test.
Although this patient developed abrupt symptoms, had no systemic symptoms and showed normal ileocecal findings in a small bowel series, we thought there was a possibility for early stage Crohn's disease localized in the small bowel, so we followed her closely. The patient has had no symptoms, and laboratory findings such as inflammatory markers, hemoglobin, and albumin have been maintained in the normal range for 18 months after eradication therapy.
There is a limitation for revealing that a peptic ulcer is associated with H. pylori infection in this article such as other reports. Nevertheless, as we experienced that the patient who did not have any systemic disease before and had a structural deformity of the duodenal bulb caused by a gastrotomy, was improved by eradication and antisecretory therapy. Therefore, we report a case of a distal duodenal and proximal jejunal ulcer associated with H. pylori infection.
Figures and Tables
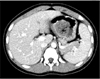 | Fig. 1Abdominal computed tomography (CT) scan shows a 4 cm sized low attenuating mass-like lesion in the stomach. |
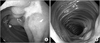 | Fig. 2The esophagogastroduodenoscopic findings. (A) Active ulcer lesion (at 60 cm from incisor) covered with whitish exudate at the base and surrounded with erythematous mucosa. (B) Multiple longitudinal ulcerative lesions beyond the second portion of the duodenum. |
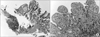 | Fig. 4(A) A shallow focal ulcerative lesion (black arrow) with superficial layers of fibrinoid necrosis, underlying granulation tissue and dense lymphoid infiltration is noted in the proximal jejunum (hematoxylin and eosin [H&E], ×100). (B) Duodenal mucosa shows severe chronic active inflammation with reactive epithelial atypia (H&E, ×200). |
References
1. Dohil R, Hassall E. Wyllie R, Hyams JS, editors. Gastritis, gastropathy and ulcer disease. Pediatric gastrointestinal and liver disease. 2006. 3rd ed. Philadelphia: Elsevier Saunders;392–407.

2. Drumm B, Day AS, Gold B, Gottrand F, Kato S, Kawakami E, et al. European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Helicobacter pylori and peptic ulcer: Working Group Report of the Second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004. 39:Suppl 2. S626–S631.
3. Macarthur C, Saunders N, Feldman W. Helicobacter pylori, gastroduodenal disease, and recurrent abdominal pain in children. JAMA. 1995. 273:729–734.

4. Seo JK. Helicobacter pylori infection in children. Korean J Pediatr Gastroenterol Nutr. 1998. 1:9–18.
5. Seo JK. Recurrent abdominal pain in children. J Korean Med Assoc. 1999. 42:859–867.
6. Yeomans ND. The ulcer sleuths: the search for the cause of peptic ulcers. J Gastroenterol Hepatol. 2011. 26:Suppl 1. 35–41.

7. Nayeb-Hashemi H, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2009. 25:537–543.

8. Graham DY, Osato MS. H. pylori in the pathogenesis of duodenal ulcer: interaction between duodenal acid load, bile, and H. pylori. Am J Gastroenterol. 2000. 95:87–91.

9. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983. 321:1273–1275.

10. Queiroz DM, Rocha GA, Mendes EN, Carvalho AS, Barbosa AJ, Oliveira CA, et al. Differences in distribution and severity of Helicobacter pylori gastritis in children and adults with duodenal ulcer disease. J Pediatr Gastroenterol Nutr. 1991. 12:178–181.

11. Bittencourt PF, Rocha GA, Penna FJ, Queiroz DM. Gastroduodenal peptic ulcer and Helicobacter pylori infection in children and adolescents. J Pediatr (Rio J). 2006. 82:325–334.
12. Hobsley M, Tovey FI, Holton J. Precise role of H. pylori in duodenal ulceration. World J Gastroenterol. 2006. 12:6413–6419.
13. Skok P. An unusual clinical course of a hormone-secreting gastrointestinal tumor: a case presentation from clinical practice. Hepatogastroenterology. 1998. 45:1655–1659.
14. Clémençon GH, Lawson HH. Enhancement of duodenal and jejunal ulceration by histamine-induced gastric hypersecretion in dogs with diversion of duodenal contents. Scand J Gastroenterol Suppl. 1984. 92:129–132.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download