Abstract
Nontyphoidal Salmonella is a type of well-known foodborne pathogen that causes gastroenteritis, bacteremia, and subsequent focal infection. Moreover, colonic ulcers, caused by nontyphoidal Salmonella infection, are considered uncommon in children. We report on the case of a 32-month-old healthy female with diffuse left-side colonic ulcers that presented with copious hematochezia, caused by a Salmonella enteritidis infection.
Nontyphoidal salmonellosis is a well-known food borne disease that is caused by a Salmonella infection and includes gastroenteritis, bacteremia, and subsequent focal infection. Salmonella infection is known to occur primarily in the small intestine and is self-limiting [1]. The risk factors for this bacterial infection include gastric hypoacidity, recent antibiotic usage, age (the very young and the very old), and an immunosuppressive condition [2].
The literature includes several reports on colonic ulcerations in adults caused by nontyphoidal Salmonella [3,4], but this type of ulceration is considered uncommon in children. Only 2 reports have demonstrated colonic ulcerative changes in children that were due to Salmonella infections [5,6]. One article reported on a 9-month-old girl with colonic ulcers and colonic perforation that were caused by Salmonella typhimurium infection [5], and the other 3 cases of Salmonella gastroenteritis had similar features similar to inflammatory bowel diseases. Endoscopic and histopathological findings demonstrated that a bacterial infection was present [6]. In this article, we report on the case of a 32-month-old healthy female with diffuse left-side colonic ulcers that were caused by a multidrug-resistant Salmonella enteritidis infection.
The patient was born at full-term gestational age with a birth weight of 2.4 kg. Neither the patient nor her immediate family members had any history of significant medical illnesses.
The patient was transferred to our hospital because she experienced uncontrolled copious hematochezia. She had been admitted to a local clinic prior to the transfer as she had high fever, cough, and rhinorrhea, which had been present for 2 days. Nonprojectile vomiting and diarrhea developed during her first day at the local hospital. Vomiting episodes occurred 3 times per day and a small volume of watery diarrhea was excreted 4-5 times per day that was accompanied with diffuse abdominal pain. However, the patient excreted bloody diarrhea during her second day. This diarrhea, which was accompanied by tenesmus and severe abdominal pain, was a mix of fresh blood and a small amount of fecal materials, and occurred 6-7 times per day.
On the day of admission, the body weight of the patient was 14.5 kg (50-75 percentile). She was irritable because of severe abdominal pain and lethargic owing to a high fever (40℃). Her blood pressure was 100/60 mmHg, her pulse rate was 90 beats/min, and her respiratory rate was 28 breaths/min. Clinically, she was considered to be severely ill. Her abdomen was distended, and focal tenderness was present in the left lower quadrant. Auscultation examinations revealed bowel sounds were lower than normal.
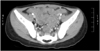
Distended bowel loops were observed on abdominal x-ray, and ultrasonography of the abdomen revealed an edematous large bowel wall. Whole-abdominal computed tomography showed diffuse wall thickening of the transverse, descending, sigmoid colon, and rectum (Fig. 1). Hematological examinations revealed that the patient had a hemoglobin level of 10.5 g/dL, a total white cell count of 20.0×109/L, a platelet count of 264×109/L and granulocyte and lymphocyte levels of 80.6 and 11.7%, respectively, with left-shifted and toxic granulations and cytoplasmic vacuoles. Her electrolytes, creatinine, and hepatic transaminase levels were normal, although her C-reactive protein level was elevated (28.5 mg/dL; normal range: approximately 0-0.5 mg/dL). Both Widal O and H titers were less than 1:20. Analysis of gas in the blood indicated mild metabolic acidosis with partially compensated respiratory alkalosis. The results of stool specimen analysis were negative for ova and parasites, as well as Clostridium difficile toxin. The stool wet smear revealed 10-19 white blood cells/high power field (HPF) and many red blood cells/HPF. The result of stool culture was positive for Salmonella group D (S. enteritidis) bacteria with resistance to ampicillin, cefepime, cefazolin, cefotaxime, ceftazidime, and piperacillin.
A sigmoidoscopy was performed on day 7 after admission as the presence of blood in the stool of the patient persisted, although her abdominal pain improved. We found diffuse ulcers in the rectum and sigmoid colon with edematous, hyperemic, and friable mucosa (Fig. 2). Biopsies demonstrated necrotic slough with fibrinous exudates containing debris and neutrophils overlying the underlying submucosa at the base of the ulcer.
After blood, urine, and stool cultures had been obtained, intravenous cefotaxime (100 mg/kg/day) was administered to the patient. Her symptoms had failed to improve after 2 days; therefore, intravenous metronidazole was added to her treatment. On day 4 after admission, cefotaxime plus metronidazole was replaced with piperacillin/tazobactam as the stool culture had demonstrated that the bacteria was resistant to antibiotics. At day 6, S. enteritidis was isolated from the stool. The patient was managed with intravenous antibiotic treatment, oral zinc sulfate (50 mg/day), and probiotics (Saccharomyces boulardii) for 2 weeks and was discharged from our hospital in good health and with no evidence of bleeding via anal verge. The 1-week and 2-month follow-up indicated that she was healthy with a culture-negative stool.
In comparison to the disease symptoms caused by Salmonella typhi, those caused by nontyphoidal Salmonella are considered less severe. In Korea, a clinical study of 72 children with salmonellosis reported that the clinical outcomes of these children were benign, although the incidence of antibiotic-resistant isolates was increased, and Salmonella group D bacteria was isolated most frequently [7]. However, Gordon and Graham [8] reported that the incidence of invasive salmonellosis has increased among children in Malawi, which is likely to be associated with the emergence of drug-resistant nontyphoidal Salmonella serovars Enteritidis and Typhimurium. In this case, S. enteritidis was isolated and was shown to cause colonic ulceration.
The 4 cases of nontyphoidal salmonellosis with colonic inflammation in children that were reported previously were caused by either S. typhimurium or S. enteritidis [5,6]. The clinical symptoms of previous reports were prolonged or repeated fever, abdominal pain, and nonbloody diarrhea with/without vomiting [5,6]. In our case, the main clinical presentation was copious hematochezia. Bloody diarrhea has been reported to occur in 14.0-41.6% of children with nontyphoidal salmonellosis in Korea [7,9]. Patients with severe Salmonella enteritis have clinical presentations similar to those of patients with inflammatory bowel diseases, such as multiple deep ulcers in the rectum; a descending colon with surrounding mucosal hyperemia and edema; or pancolitis with marked mucosal edema, erythema, loss of haustral folds, and superficial ulcerations throughout the colon [6]. In this case, the endoscopic findings revealed diffuse ulcers in the rectum and sigmoid colon, which were surrounded by edematous and hyperemic mucosa and were easily bruised. Treating patients with symptoms of nontyphoidal Salmonella infection with antimicrobial agents is not recommended owing to the self-limiting nature of the illness, as well as the association between prolonged antibiotic use and the carrier state for the organism [10]. Antimicrobial therapy should be initiated for those patients who are severely ill or with risk factors for extraintestinal spread of infection (e.g., immunocompromised patients, infants aged less than 3 months old, and hemolytic anemia patients) [6,9]. In the present case, antibiotics were administered owing to suspected bacterial enterocolitis caused by Shigella or Escherichia coli (symptoms of high fever, abdominal tenderness, and bloody diarrhea).
The antibiotics that are often administered for Salmonella infection are fluoroquinolones, ampicillin, or third-generation cephalosporins. The recent Korean Nationwide Surveillance Study for Non-Typhoidal Salmonella reported that resistance to trimethoprim-sulfamethoxazole and ampicillin is common, and therefore, the use of a third-generation cephalosporin or quinolone is recommended if the drug susceptibility of the bacteria is unknown [11]. Currently, studies are being conducted to quantify the efficacy of fluoroquinolones in children in areas of the world where multidrug-resistant S. typhimurim is common [12]. Resistance to third-generation cephalosporins is likely to be an increasing clinical problem, especially among children, since these agents are the current drugs of choice for the treatment of bacterial infection [6]. In addition, resistance to third-generation cephalosporins and aminocoglycosides has been reported; however, the bacteria are mainly susceptible to imipenem and meropenem [13] In this case, the S. enteritidis that was isolated from the stool culture was resistant to third-generation cephalosporin, and the initial antibiotic, cefotaxime, administered for treatment was ineffective. After treatment with piperacillin/tazobactam, abdominal pain and the degree of hematochezia improved rapidly and the bacteria were found to be absent from the stool samples.
Nontyphoidal salmonellosis occurs more frequently during the summer months [7] and it may be related to food-borne transmission. The common clinical symptoms of salmonellosis are similar to the symptoms of viral gastroenteritis, such as vomiting, watery diarrhea, and fever [14]. Hand-washing and the strict control of carrier are important for the prevention of salmonellosis.
The present case suggested that nontyphoidal salmonellosis can cause colonic ulcers in children and antibiotic-resistant nontyphoidal Salmonella infection might be associated with the inflammation of the colonic mucosa.
Figures and Tables
References
3. Tedesco FJ, Hardin RD, Harper RN, Edwards BH. Infectious colitis endoscopically simulating inflammatory bowel disease: a prospective evaluation. Gastrointest Endosc. 1983. 29:195–197.

4. Vender RJ, Marignani P. Salmonella colitis presenting as a segmental colitis resembling Crohn's disease. Dig Dis Sci. 1983. 28:848–851.

5. Chui CH, Joseph VT, Chong CY. Salmonella typhimurium: a rare cause of colonic ulceration and perforation in infancy. J Pediatr Surg. 2000. 35:1494–1495.

6. Friesen C, Hill I, Woods C. Salmonella gastroenteritis mimicking onset of inflammatory bowel disease in children. J Pediatr Gastroenterol Nutr. 2008. 46:84–86.

7. Noh SH, Yu KY, Kim JS, Hwang PH, Jo DS. Salmonellosis in children: analysis of 72 salmonella-positive culture cases during the last 10 years. Korean J Pediatr. 2009. 52:791–797.

9. Na SY, Kim BC, Yang HR, Jung SJ, Lee KH, Ko JS, et al. Non-typhoidal salmonella gastroenteritis in childhood: clinical features and antibiotics resistance. Korean J Pediatr Gastroenterol Nutr. 2002. 5:150–157.

10. Nelson JD, Kusmiesz H, Jackson LH, Woodman E. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics. 1980. 65:1125–1130.

11. Chung HS, Lee H, Lee Y, Yong D, Jeong SH, Lee BK, et al. A Korean nationwide surveillance study for non-typhoidal salmonella isolated in humans and food animals from 2006 to 2008: extended-spectrum beta-lactamase, plasmid-mediated AmpC beta-lactamase, and plasmid-mediated quinolone resistance qnr genes. Korean J Clin Microbiol. 2012. 15:14–20.

12. Wain J, Hoa NT, Chinh NT, Vinh H, Everett MJ, Diep TS, et al. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin Infect Dis. 1997. 25:1404–1410.

13. Maiorini E, Lopez EL, Morrow AL, Ramirez F, Procopio A, Furmanski S, et al. Multiply resistant nontyphoidal Salmonella gastroenteritis in children. Pediatr Infect Dis J. 1993. 12:139–145.
14. Lim HS. Changing patterns of communicable diseases in Korea. J Prev Med Public Health. 2005. 38:117–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download