Abstract
Toxic hepatitis is a rare but devastating disease in children. Herbs are widely used in oriental medicine to treat various symptoms in Korea, however, several herbs have been reported to induce liver injury. We report a case of toxic hepatitis induced by Hovenia dulcis in a 3-year-old boy. He complained of nausea, abdominal discomfort, and jaundice. The patient had consumed water boiled with hovenia dulcis for about 1 year prior to presentation. A diagnosis of toxic hepatitis was made based on his history, laboratory data, viral markers, ultrasonography, and biopsied liver tissue. We administered supportive management for acute fulminant hepatitis but his symptoms and liver function progressed. He was transferred to another hospital for further evaluation and consideration for liver transplantation. Because acute liver failure due to herbs or dietary supplement taken for a long time is often fetal, it is important to make early diagnosis and stop taking the drug as soon as drug induced liver injury is suspected.
Drug induced liver injury is being increasingly recognized as a cause of clinically significant acute and chronic liver diseases. Such cases may lead to non-specific degeneration and even fulminant hepatic failure. In pathology, drug induced liver injury may appear as hepatic cell inflammation or cholestasis or a mixed condition of both [1,2].
In Korea, as many people are highly concerned about health and tend to engage in taking health supplements, toxic hepatitis accounts for much cases of acute hepatitis [3]. It was reported that adults showed a frequency of toxic hepatitis out of all cases of hepatitis in Korea ranging from 2% to 40%, while children showed approximately 15%, and oriental medicine was assumed to be the most common cause of toxic hepatitis [3,4]. Many Korean children face imprudent use of a variety of oriental medicine given by their parents for the purpose of better growth and physical constitution improvement, and for this reason the frequency of toxic hepatitis seems actually higher than the previous report.
Hovenia dulcis (Oriental Raisin Tree) is a deciduous large-leaved tree belonging to Rhamnaceae. Its fruit and seeds are used as oriental medicine called 'Jiguja'. Recently, consecutively reports have indicated that Polysaccharide HD-1, extracted from the fruit stalk, is effective in preventing liver cirrhosis and protecting the liver, further increasing the application of hovenia dulcis in Korea [5]. However, there hasn't been many studies on pharmacokinetics, internal metabolism, proper usage and side effects of hovenia dulcis. In Korea, two cases of toxic hepatitis have been reported in adult patients after ingesting hovenia dulcis [3,6], but there has not yet been any report on toxic hepatitis in children.
In this article, we report a case of toxic hepatitis induced by consuming hovenia dulcis-decocted water every day for 1 year in a 3-year-old boy.
Patient: 3 year and 6 month-old boy
Chief complaint: Jaundice
Present illness: A previously healthy child came to a local hospital because of jaundice. He complained of nausea, coke-colored urine for 10 days, jaundice in the sclera and all over the body for 7 days. He has been taking hovenia dulcis-decocted water every day for 1 year. Hematologic and blood chemical values were as follows: Hb 10.3 g/dL, WBC 4.300/mm3, AST 1,643 IU/L, ALT 1,813 IU/L, bilirubin (total/direct) 7.2/6.0 mg/dL, Ammonia 101 µg/dL, PT 10.8 sec, aPTT 26.4 sec, PT-INR 0.94, r-GTP 122 IU/L. Conservative treatment was performed while he was being hospitalized, but his symptoms became more serious, and the blood test worsened to AST 1,753 IU/L, ALT 1,232 IU/L, bilirubin (total/direct) 10.3/8.6 mg/dL on the 2nd day after admission, so he was transferred to our hospital.
Past medical history and family history: He was born, weighing 3,700 g, by spontaneous vaginal delivery at 38 weeks of gestation. He had no previous medical history. His family had some medical history related to liver diseases; grandfather died from liver cirrhosis, his father had a fatty liver and his mother was a hepatitis B carrier.
Physical examination: He was 94 cm (3-10 percentile) in height and 14.6 kg (10-25 percentile) in weight and vital signs were: blood pressure of 100/60 mmHg, pulse of 118 beats per minute, respiratory rate of 24 breaths/minute, and temperature of 37.2℃. On admission, physical examination showed that he had a clear mental status but seemed acutely ill with jaundice in the sclera and all over the skin. Abdominal distention was not observed, but the liver was promoted to the size of 3.8 cm (2 finger-breadth) with its border formed gently but firmly under the right rib, without splenomegaly. Direct tenderness was found on the diffuse abdomen with no rebound tenderness.
Laboratory investigations: On admission, initial laboratory test showed that Hb 9.7 g/dL, WBC 3,400/mm3 (seg 11.5%, lymphocyte 78.5%), platelet 218,000/mm3, and eosinophil 0.4%. The biochemical profile consisted of AST 1,627 IU/L, ALT 1,113 IU/L, AST/ALT ratio 1.46, bilirubin (total/direct) 14.56/9.63 mg/dL, Ammonia 54 µg/dL, r-GTP 40 IU/L, and ALP 336 IU/L, and blood coagulation test showed PT 11.6 sec, aPTT 34.2 sec, PT- INR 1.03. The RUCAM ratio (ALT value/ALT upper limit of normal)/(ALP value/ALP upper limit of normal) was approximately 24. These tests indicated hepatocellular liver injury [7].
Serum test was conducted but there was no particular evidence good enough to prove hepatitis. Serologic testing for hepatotropic viruses was all negative for: HBs Ag, anti-HBcIgM Ab, anti-HAV IgM, anti-HCV Ab, CMV IgM, EBV viral capsid IgM, and HSV IgM. Immunologic profile revealed no pathologic auto antibodies; anti-nuclear antibody, antimitochondria antibody, anti-smooth muscle antibody, and anti liver-kidney-microsomes antibody were all negative. There was no bacterial growth in the blood, urine and feces cultures. Ceruloplasmin, α1-antitrypsin and ferritin levels were normal. In addition, PFIC gene ABCB11 and ATP8B1 were both negative, and it was also found negative from the Anti-HAV IgM continuous test.
Radiologic findings: There was nothing unusual in the simple abdominal X-ray. In the ultrasonographic examination of the abdomen, slight hepatomegaly and several enlarged lymph nodes below 1 cm were observed around the hilar area with gallbladder hyperplasia and a small amount of ascites.
Liver biopsy findings: On the second day after admission, a needle liver biopsy was performed. Histological examination showed severe hepatocytic degeneration with frequent acidophilic bodies and balloon change, apoptosis, severe lobular activity and moderate porto-periportal activity. There were frequent spotty inflammatory collections in the hepatic lobule and some cholestasis. In addition, the portal tracts showed periportal fibrosis with some lymphoid infiltration of eosinophils and a few neutrophils, all of which indicate toxic hepatitis (Fig. 2).
Treatment and Outcome: Any other factors such as virus, metabolic liver disease, autoimmune hepatitis were not found to bring about the liver injury. Based on the point of RUCAM Scale 6, he was supplied with sufficient calories and conservative treatment (fat soluble vitamin, Ursodesoxycholic acid, Biphenyl dimethyl dicarboxylate, lactulose, prophylactic antibiotics-amoxicillin+clavulanate) under the diagnosis of toxic hepatitis. Laboratory tests were performed on a regular basis (Fig. 3).
Despite the conservative treatment, his anorexia, weakness and jaundice didn't improve and coke-colored urine continued. On the 8th day after admission, abdominal distension and diffuse abdominal pain became more serious, and on the 9th day of hospitalization, it worsened to AST 2,362 IU/L, ALT 1,130 IU/L, AST/ALT ratio 2.09, bilirubin (total/direct) 20.2/13.49 mg/dL, Ammonia 113 µg/dL, PT 15.0 sec, aPTT 39.5 sec, and PT-INR 1.32, so he was transferred to another hospital for further evaluation and liver transplantation.
In Korea, since people believe natural herb medicine has less side effects than commercialized medicine, the use of oriental medicine and health supplements is increasing, and the abuse of such product is more likely to increase the frequency of liver injury [8,9]. According to a recent retrospective research on 159 patients hospitalized for toxic hepatitis, 41.5% was caused by oriental medicine and 24.5% was by folk drugs such as Dictamnus, Perennial Artemisia, Ginseng, kudzu and Sanghwang Mushroom [3].
Efficacy and safety of all medicines should be secured including herb medicine and health supplements since overuse or misuse can lead to development of diseases. Not only do their side effects appear as toxic hepatitis but occur in various different forms. However, since most bioactive substances are metabolized in the liver, toxic hepatitis is a side effect that should always be examined for crude drugs or health medicine in addition to all other medicine [8,10]. Especially, children have different physiological characteristics from those of adults and are more sensitive to medicine. For this reason, it is even more difficult to predict the outcome, so it is important to attain information about the drug and its side effects on children.
As a deciduous large-leaved tree belonging to Rhamnaceae, Oriental Raisin Tree (Hovenia Dulcis) is called 'Hoggae Tree', and its fruit and seeds are used as oriental medicine called 'Jiguja'. It is reported that its fruit increases ADH (Alcohol dehydrogenase) and ALDH (Acetaldehyde dehydrogenase) activity which accelerates alcoholysis inside the body, further impeding the oxidation of toxic substances. Polysaccharide HD-1, extracted from the fruit stalk, is known to be effective in preventing liver cirrhosis and protecting the liver, which leads to an increased use of oriental raisin trees in Korea [5,11]. However, there have been two cases of toxic hepatitis in adults, in addition to the present case, in which other factors, such as virus, metabolic liver diseases and autoimmune hepatitis, were not observed as a cause of liver injury. As a result, hovenia dulcis seemed to be the cause for the degeneration of hepatic functions.
Since biochemical index for the diagnosis of toxic liver injury has not been established and it is not ethically allowed to put the injured liver cell into the body, the evaluation is mostly based on the causality assessment method, especially the RUCAM (Roussel Uclaf Causality Assessment Method) scale [3,7,12]. In this case, the patient showed hepatocellular-type liver injury with R value ≥5. Based on the RUCAM scale, this case was classified under 'probable' with a total of 6 points in combination of the following factors: time to onset from the beginning (compatible, +1), time to onset from cessation (compatible,+1), course (compatible, +1), risk factors (+0), concomitant drugs (+0), search for non-drug courses (+2), previous information on hepatotoxicity of the drug (+1), and response to readministration (+0).
Since 2003, a prospective study DILIN (Drug-Induced Liver Injury Network) has been conducted in the US and it defines the legal term-used in descriptions and possibility (%) of scores obtained from RUCAM. The patient in the present case belonged to 'Probable' with 50 to 74% of possibility, and the causality is supported by 'the preponderance of evidence' [7,13]. However, only few previous reports have used RUCAM as a diagnosis tool to investigate the cause of toxic hepatitis due to herbal medicine, health supplements or folk remedies. As a result, the frequency is evaluated lower than its actual value. Besides, there are many cases of patients who take those alternative medical products in a low concentration for a long time, which increases the time between the medicine intake completion and the symptom manifestation. Therefore, it is required to regulate the RUCAM Scale fit for the described circumstances in Korea in the future [7].
Drug-induced toxic hepatitis can be divided into intrinsic hepatotoxicity, occurring to most of the people who are exposed to the toxic substance in proportion to the dosage used, and idiosyncratic hepatotoxicity, occurring to some of the people who are exposed to the toxic substance with little or no dosage dependence [14,15]. Intrinsic hepatotoxicity is mostly mediated by cytomchrome P450-induced type 1 reaction metabolome and free radicals occurring when the bile acid movement protein is impeded which lead to the damage of cell membrane and activates apoptosis [1]. On the other hand, idiosyncratic reactions in certain patients can reflect aberrant pathways of drug metabolism, possibly related to genetic polymorphisms presenting with irregular manifestations of individuals' liver injury and poor prognosis since it is usually revealed after 4 to 6 weeks [16]. As a mechanism of idiosyncratic reaction-induced hepatitis, immunologic factors are suggested. Cytochrome P450-metabolized substance forms neo-antigens by combining with proteins inside the liver, and the secondary change of plasma membranes leads to new antigenicity, further reacting with the immune system. As a result, the disease is not improved but sustained or even worsened, and leads to a chronic process in some cases [1,3,17]. In the present case, as the patient didn't show hypersensitive reactions such as fever, rash, arthralgia and eosinophilia, a steroid treatment was not performed, implicating that his toxic hepatitis was mainly based on idiosyncratic reactions [3,4].
In this case, the patient had taken hovenia dulcis-decocted water over 1 year, and as an intrinsic and idiosyncratic reaction-induced mechanism, he had an injury of the liver. In the liver biopsy, widespread hepatic cell degeneration was observed, accompanied with ballooning degeneration in addition to the deposition of lymphocytes, neutrophils and eosinophils and replacement of some liver cells with connective tissues. Despite conservative treatment, toxic hepatitis developed into hepatic failure.
Hovenia dulcis is widely used since it can be obtained relatively easily, and many think it would help improve liver functions. It is not clear what components of hovenia dulcis have relations with what side effects, or if the toxic hepatitis is caused by the overuse or pollutants, such as agricultural chemicals. However, it is very important to carry out further research on the use of hovenia dulcis and actively promote people's awareness of the effects of its misuse. One of the effects of taking health supplements or herbal medicine for a long time is toxic hepatitis which can develop into hepatic failure requiring liver transplantation. Also, idiosyncratic reaction-induced toxic hepatitis presents with poor prognoses, so it is more important than anything else to carry out a preventive treatment of being cautious in the initial phase and stop taking alternative medical products [16].
Drug-induced hepatitis is one of the causes of acute hepatitis. For children, an imprudent use of various oriental medicine has been increasing for better growth and physical constitution improvement leading to increased frequency of herbal medicine-related toxic hepatitis although it has not been clearly reported yet.
In this study, we performed conservative treatment for a 3 year and 6 month-old male child with fulminant hepatitis, who had been taking hovenia dulcis-decocted water for over 1 year, which was known to be efficacious in liver disorders. However, the liver injury continued and the findings are presented in this report.
Figures and Tables
 | Fig. 1Abdominal ultrasonography of the case show. (A) Several enlarged lymph nodes of less than 1 cm at porta hepatitis. (B) Minimal effusion of perihepatic andperisplenic area, and (C) GB wall edema. |
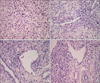 | Fig. 2Pathology findings of needle biopsied liver tissue show. (A) Some hepatocytic degeneration with frequent acidophilic bodies and balloon change (H&E, ×200). (B) Frequent spotty inflammatory collections in the hepatic lobule (H&E, ×200). (C, D) Some periportal fibrosis with some lymphoid infiltration, eosinophils and a few neutrophils (H&E, ×200). |
References
1. Kang HS, Choi HS, Yun TJ, Lee KG, Seo YS, Yeon JE, et al. A case of acute cholestatic hepatitis induced by Corydalis spediosa Max. The Korean Journal of Hepatology. 2009. 15:517–523.
3. Kang SH, Kim JI, Jeong KH, Ko KH, Ko PG, Hwang SW, et al. Clinical characteristics of 159 cases of acute toxic hepatitis. The Korean Journal of Hepatology. 2008. 14:483–492.

5. Fang HL, Lin HY, Chan MC, Lin WL. Treatment of chronic liver injuries in mice by oral administration of ethanolic extract of the fruit of Hovenia dulcis. Am J Chin Med. 2007. 35:693–703.

6. Sohn CH, Cha MI, Oh BJ, Yeo WH, Lee JH, Kim W, et al. Liver transplantation for acute toxic hepatitis due to Herbal medicines and preparations. Journal of the Korean Society of Clinical Toxicology. 2008. 6:110–116.
7. Kim DJ. The assessment of toxic liver injury. The Korean Journal of Gastroenterology. 2009. 53:5–14.
8. Bae SH, Kim DH, Bae YS, Lee KJ, Kim DW, Yoon JH, et al. Toxic hepatitis associated with Polygonimultiflori. The Korean Journal of Hepatology. 2010. 16:182–186.
9. Bénichou C. Criteria of drug-induced liver disorders. Report of an international meeting. J Hepatol. 1990. 11:272–276.
11. Hyun TK, Eom SH, Yu CY, Roitsch T. Hoveniadulcis-an Asian traditional herb. Planta Med. 2010. 76:943–949.
12. An SY, Cheong JY, Kim SS, Lee DM, Seok JY, Kim YB, et al. One case of fulminant hepatic failure related to Dictamnusdasycarpus. Korean Journal of Medicine. 2010. 78:490–494.
13. Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug-induced liver injury network (DILIN) prospective study: rationale, design and conduct. Drug Safety. 2009. 32:55–68.

14. Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury:mechanisms and test systems. Hepatology. 2001. 33:1009–1013.

16. Shim JO. Diagnosis and management of acute liver failure in children. Korean J Pediatr Gastroenterol Nutr. 2008. 11:Suppl 2. 50–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download