Abstract
Gastric ulcers are rare in children and are typically seen in cases of Helicobacter pylori (H. pylori) infection, non-steroidal anti-inflammatory drugs (NSAIDs) use, and critical illnesses such as sepsis. The risk of a bleeding ulcer due to use of NSAIDs is dependent on the dose, duration, and the individual NSAIDs, but the bleeding may occur soon after the initiation of NSAID therapy. An experience is described of a 16-month-old infant with a bleeding gastric ulcer after taking the usual dosage of ibuprofen for 3 days. The infant was also successfully treated with endoscopic hemostasis. Even a small amount of ibuprofen may be associated with bleeding gastric ulcers in infant.
Although peptic ulcers are rare in children, they can occur at any age. H. pylori infection is the major cause of primary peptic ulcers. Secondary ulcers are usually associated with systemic illness such as sepsis, head trauma, burns, and drugs (e.g. NSAIDs, corticosteroids, sodium valproate, theophylline) [1]. There has been a profound change in peptic ulcer disease in Western countries in the last decade. The prevalence of H. pylori positive ulcers has declined, and NSAIDs-induced ulcers has emerged in Western countries in adults. Among the NSAIDs, Ibuprofen is widely used as antipyretic agents in children.
Non-selective NSAIDs increase the risk compared with non-users of peptic ulcer disease by a factor of 5 [2], and upper GI bleeding by a factor of 4 [2,3]. Established risk factors for NSAID-related upper GI bleeding include previous complicated ulcers, multiple NSAID use, high-doses of NSAIDs, previous uncomplicated ulcers, concomitant use of anticoagulants, age >60 years, and concomitant use of corticosteroids [3-6].
The risk of an ulcer bleeding due to use of NSAIDs is dependent on the dose, duration, and the individual NSAIDs. An experience with a 16-month-old infant is described, who suffered from ibuprofen-induced bleeding gastric ulcer and had undergone endoscopic hemostasis using epinephrine injection and electro-coagulation.
A 16-month-old female presented to the emergency department (ED) with repetitive vomiting for 2 days, and hematemesis 3 times on the day of presentation. A week prior to the ER presentation, the patient was seen at a nearby hospital for 3 days of fever and poor oral intake, given an acute diagnosis of acute tonsillitis and treated with antibiotics and ibuprofen. The patient had neither an abnormal past medical nor a familial history.
Vital signs were measured as blood pressure 130/90 mm Hg, pulse rate 60/min, respiratory rate 20/min, and body temperature 37.3℃. The patient subsequently did not show any signs of irritability or lethargy. Her conjunctivae were not pale. Bowel sounds were normoactive with no abdominal distention, tenderness, guarding or rebound tenderness. The patient had neither hepatosplenomegaly nor palpable abdominal masses.
A peripheral blood test revealed hemoglobin 12.8 g/dL, hematocrit 36.5%, white blood cell count 9,480/mm3, and platelet count 210,000/mm3. The blood chemistry was also analyzed as total protein 6.5 g/dL, albumin 4.2 g/dL, total bilirubin 0.2 mg/dL, AST 66 IU/L, ALT 22 IU/L, alkaline phosphatase 174 IU/L, blood urea nitrogen 9.8 mg/dL, creatinine 0.4 mg/dL, ammonia 61 µg/dL, and CRP 0.33 mg/dL. A blood coagulation test revealed PT/aPTT of 10.7/38.8 seconds. A routine radiologic exam of the chest and abdomen revealed no abnormalities. The plasma gastrin level was 57.1 pg/mL (<110 pg/mL). The serum anti-H. pylori IgG was negative and the H. pylori stool antigen test showed a negative result.
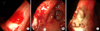
An esophago-gastro-duodenoscopy (EGD) (GIF 160, diameter 8.6 mm with biopsy channel 2.8 mm; Olympus Optical, Co., Ldt., Tokyo, Japan) was performed after confirmation of active bleeding via a Levin tube irrigation. A bleeding ulcer was noted at the lesser curvature near the gastric angle. The ulcer was measured approximately 10 mm in diameter, was triangular shape, and had blood oozing from the exposed vessel inside the ulcer (Fig. 1). A grounding plate was placed on the patient's thigh. A Bolus of 1.5 mL of a 1:10,000 solution of epinephrine were injected into the submucosa, and the bleeding slowed. Immediately after the epinephrine injection, electrocoagulation was performed by using polypectomy snare (SnareMaster SD-210U-25; Olympus Optical, Co., Ldt., Tokyo, Japan). Three successive pulses of 20 watt for 1 to 2 seconds was applied through the tip of the snare around and then on the oozing vessel, until a white coagulum appeared. Thereafter, complete hemostasis was achieved. The treated site was observed for one minute to ensure a no further bleeding or complication.
The patient was placed on lansoprazole and sucralfate, kept fasting, with the exception of medications for 24 hours following the procedure. The patient was closely observed and had no further hematemesis. There was no evidence of rebleeding for seven days after the treatment. The patient was discharged on the 7th hospital day, and a follow up EGD was performed after being treated with lansoprazole for 8 weeks. On a follow up EGD, the previous ulcer healed to stage S2 and mucosal biopsies were obtained from the gastric antrum. The patient's CLO test (rapid urease test) was negative for evidence of H. pylori. Histological examin-ation of biopsy specimens confirmed no presence of H. pylori.
Upper gastrointestinal bleeding (UGIB) can potentially be life-threatening. In adults, peptic ulcer bleeding is the most common manifestation of UGIB, accounting for approximately 50% [7]. The incidence of peptic ulcer bleeding varies from 20 to 50 patients per 100,000 people per year in adults [8]. In contrast, peptic ulcer disease in children is rare. The estimated incidence of peptic ulcer bleeding in the US pediatric population in 2008 ranged from 0.5 to 4.4 per 100,000 individuals according to an epidemiologic study [9].
The two most important causes of peptic ulcer disease are H. pylori infection and NSAIDs, accounting for more than 90% [10]. Other causes include critical systemic illness such as sepsis, head trauma, and burns.
Regrettably for the case of the 16-month-old infant, gastric biopsy for H. pylori tests could not be done, since emergency endoscopic hemostasis had been performed in the patient with unsatisfactory sedation. Nevertheless, H. pylori infection could be ruled out as a cause of gastric ulcer in the patient, considering the fact that serum anti-H. pylori IgG and the result of the H. pylori stool antigen test were both negative, and on a follow up EGD, the CLO test (rapid urease test) and histological examination of biopsy specimens revealed no evidence of H. pylori infection. It is safe to say that NSAIDs are the cause of gastric ulcer in the patient, considering that the patient was a previously healthy infant, and that acute tonsillitis is not sufficiently critical illness to cause gastric ulcer.
Non-selective NSAIDs increase the risk of peptic ulcer disease by a factor of 5 compared with non-users [2]. NSAIDs cause gastroduodenal damage by topical injury to the mucosa and by systemic inhibition of cytoprotective prostaglandins through inhibition of cyclooxygenase activity [11]. The latter appear to have the predominant role.
The use of NSAIDs or aspirin is the most important risk factor for bleeding ulcers. The risk of upper GI bleeding is 4 times greater for non-selective NSAIDs users compared with non-users [2,3]. A study suggested that NSAIDs were used by 52% of patients with bleeding ulcers; in contrast, the rate of H. pylori infection was only 43% [12].
The risk of bleeding due to NSAID use is dependent on the dose, duration, and the individual NSAIDs. One meta-analysis revealed that ibuprofen (RR 1.9; 95% CI 1.6-2.2) had the lowest relative risk, followed by diclofenac, sulindac, naproxen sodium, indomethacin, and ketoprofen. Piroxicam (RR 6.3; 95% CI 5.5-7.2) had the highest relative risk [3]. In another meta-analysis also found that Ibuprofen had the lowest relative risk, but found azapropazone to have the the greatest, and higher doses of ibuprofen had intermediate risk [13]. Among non-aspirin-NSAIDs in a case-control study [14], aceclofenac (RR 3.1; 95% CI 2.3-4.2) was associated with the lowest relative risk, whereas ketorolac (RR 14.4; 95% CI 5.2-39.9) was associated with the highest, and ibuprofen had intermediate risk (RR 4.1; 95% CI 3.1-5.3).
The risk of an ulcer [15] and UGI bleeding [16] due to NSAID use is dependent on the dose. It has been verified a clear dose-response relationship between the dosage of NSAIDs and peptic ulcer bleeding.
Notably, the first month of treatment is the period of highest risk for bleeding ulcer, with reported relative risks of 7.6 (95% CI 6.0-9.5) and 5.7 (95% CI 4.9-6.6) in some studies [3,13].
A week prior to the ER presentation, the patient took ibuprofen as a fever reliever 4 times per day for 3 days. Ibuprofen has been widely known among non-selective NSAIDs to have the lowest risk of bleeding ulcer. A study analyzing many randomized clinical trials and observational research showed that even the first dose of nonselective NSAIDs can cause bleeding ulcer [16]. It was also shown that even one dose of aspirin could cause multiple petechial hemorrhages in the fundus and antrum in as little as one hour, with visibility persisting at 24 hours in 80% of 15 normal healthy volunteers who had abnormal baseline endoscopy [17]. Based on this, it is reasonable to conclude that ibuprofen may be associated with bleeding ulcer in the patient.
Meanwhile, there has been a controversy about whether H. pylori infection plays a role in NSAID-induced peptic ulcer bleeding [18]. A meta-analysis indicated a synergy effect between H. pylori infection and NSAIDs on the development of complicated and uncomplicated ulcers [19]. This contrasts to a negative interaction between H. pylori and NSAID use in patients with bleeding ulcers compared to those with non-bleeding ulcer in one study [20].
In this patient, it would be possible to differentiate gastric ulcer from a Dieulafoy's lesion. The characteristic endoscopic findings of a Dieulafoy's lesion are an isolated protruding vessel surrounded by normal mucosa, which does not have an associated ulcer [21,22]. As described in detail, the agreed diagnostic criteria for Dieulafoy's lesion on endoscopy are the following: (1) active arterial spurting or micropulsatile streaming of blood from a minute (<3 mm) mucosal defect or through normal surrounding mucosa; (2) visualization of a protruding vessel, with or without active bleeding, within a minute mucosal defect or through normal surrounding mucosa; or (3) fresh, densely adherent clot with a narrow point of attachment to a minute mucosal defect or to normal-appearing mucosa [23,24]. Thus, a Dieulafoy's lesion could be excluded in that an oozing vessel with surrounding mucosal ulcer was revealed on an EGD in this patient.
Endoscopic hemostatic therapy for bleeding peptic ulcer includes injection methods (normal saline, vasoconstrictor as epinephrine, sclerotherapeutic agents, thrombin cocktail, or combination therapy), coagulation (monopolar, bipolar electrocoagulation, contact thermal methods using heater probe, and noncontact thermal methods using argon plasma), and a mechanical method using hemostatic clips [25,26]. An additional coagulation devices are the hot biopsy forceps and the polypectomy snares. Polypectomy snares usually consist of a monopolar wire loop electrode and all snares are designed for use with electocautery. Monopolar electrocautery means that the electrical circuit runs through the patient body to a grounding pad placed on the patient [27]. In electrocoagulation, the electric energy is converted to heat energy within the target tissue, which coagulates the tissue, causing collagen contraction and vessel shrinkage [25]. A review of electrocautery suggested using coagulation at a setting of 20 Watts for hot snaring [28]. For hot biopsy forceps, the electrosurgical generator unit is set on coagulation at a setting of 10 to 15 Watts for 1 to 2 seconds in the cecum and ascending colon, or up to 15 to 20 Watts for 2 seconds in the left colon [25,28]. Higher settings or longer application times have been associated with an increased risk of perforation.
Although the combination of injection therapy with either a mechanical or thermal method was recommended to achieve permanent hemostasis [26], it is uncertain that it had superiority over thermal or mechanical monotherapy [29]. In the patient, combined epinephrine injection and electrocoagulation by using polypecto-my snare achieved complete hemostasis without complications.
The risk of rebleeding was assessed on the endoscopic appearance of the bleeding ulcer (ulcers with signs of active spurting (Forrest Ia) or oozing (Forrest Ib) and ulcers with a nonbleeding visible vessel (Forrest IIa) have a 50% risk for rebleeding) [7,16]. It has been demonstrated that a PPI has been effective not only in controlling bleeding but also in reducing the rate of rebleeding. Therefore, a combination therapy of endoscopic hemostasis with administration of PPI was recommended [30]. The patient was placed on a PPI after endoscopic hemostasis and there was no evidence of rebleeding. The patient continued to take medication for gastric ulcer, which had healed completely on a follow up EGD.
In conclusion, even a small amount of ibuprofen may be associated with bleeding gastric ulcer in a 16-month-old infant without any other risk factors for gastric ulcer.
Figures and Tables
References
2. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic ulcer disease: a meta-analysis. Lancet. 2002. 359:14–22.

3. Hernández-Diaz S, Garcia-Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. An overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000. 160:2093–2099.

4. Pérez Gutthann S, GarciáRodríguez LA, Raiford DS. Individual nonsteroidal anti-inflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997. 8:18–24.

5. Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010. 24:121–132.

6. Langman MJ, Weil J, Wainwright P, Lawson DH, Rawlins MD, Logan RF, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994. 343:1075–1078.

7. Holster IL, Kuipers EJ. Update on the endoscopic management of peptic ulcer bleeding. Curr Gastroenterol Rep. 2011. 13:525–531.

8. Cappell MS. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010. 7:214–229.

9. Brown K, Lundborg P, Levinson J, Yang H. The incidence of peptic ulcer bleeding in the US pediatric population. J Pediatr Gastroenterol Nutr. 2012. 54:733–736.
10. Van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008. 22:209–224.

11. Park JH. Medical treatment of peptic ulcer disease in children: role of H2RA, PPI. Korean J Pediatr Gastroenterol Nutr. 2007. 10:1 Suppl. 43S–52S.
12. Ramsoekh D, Van Leerdam ME, Rauws EAJ, Tygat GN. Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use and Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2005. 3:859–864.

13. Henry D, Lim LL, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, Griffin M, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996. 312:1563–1566.

14. Lanas A, García-Rodríguez LA, Arroyo MT, Gomollón F, Feu F, González-Pérez A, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective COX-2 inhibitors, traditional non-aspirin NSAIDs, aspirin, and combinations. Gut. 2006. 55:1731–1738.

15. Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med. 1991. 114:257–263.

16. Hunt RH, Lanas A, Stichtenoth DO, Scarpignato C. Myths and facts in the use of antiinflammatory drugs. Ann Med. 2009. 41:423–437.

17. O'Laughlin JC, Hoftiezer JW, Ivey KJ. Effect of aspirin on the human stomach in normals: endoscopic comparison of damage produced one hour, 24 hours, and 2 weeks after administration. Scand J Gastroenterol Suppl. 1981. 67:211–214.
18. Manguso F, Riccio E, Nucci G, Aiezza ML, Amato G, Degl'Innocenti L, et al. Helicobacter pylori infection in bleeding peptic ulcer patients after non-steroidal antiinflammatory drug consumption. World J Gastroenterol. 2011. 17:4509–4516.

19. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic ulcer disease: a meta-analysis. Lancet. 2002. 359:14–22.

20. Okan A, Tankurt E, Aslan BU, Akpinar H, Simsek I, Gonen O. Relationship between non-steroidal anti-inflammatory drug use and Helicobacter pylori infection in bleeding or uncomplicated peptic ulcers: A case-control study. J Gastroenterol Hepatol. 2003. 18:18–25.

21. Baxter M, Aly EH. Dieulafoy's lesion: current trends in diagnosis and management. Ann R Coll Surg Engl. 2010. 92:548–554.

23. Lee YT, Walmsley RS, Leong RW, Sung JJ. Dieulafoy's lesion. Gastrointest Endosc. 2003. 58:236–243.

24. Senger JL, Kanthan R. The evolution of Dieulafoy's lesion since 1897: then and now-a journey through the lens of a pediatric lesion with literature review. Gastroenterol Res Pract. 2012. 2012:432517.

25. Kay MH, Wyllie R. Therapeutic endoscopy for nonvariceal gastrointestinal bleeding. J Pediatr Gastroenterol Nutr. 2007. 45:157–171.

26. Aabakken L. Current endoscopic and pharmacological therapy of peptic ulcer bleeding. Best Pract Res Clin Gastroenterol. 2008. 22:243–259.

27. Fyock CJ, Draganov PV. Colonoscopic polypectomy and associated techniques. World J Gastroenterol. 2010. 16:3630–3637.

28. Morris ML, Tucker RD, Baron TH, Song LM. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol. 2009. 104:1563–1574.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download