Abstract
Epilepsy surgery is classified into two types: curative epilepsy surgery and palliative surgery. The most frequently performed curative epilepsy surgery is an anterior temporal lobectomy with amygdalohippocampectomy (ATL with AH). ATL with AH includes the resection of epileptic hippocampus/amygdala and anterior temporal lobe (3~4cm from temporal pole) and is performed for treating drug refractory mesial temporal lobe epilepsy. A literature reports that more resection of epileptic hippocampus had a better surgical outcome. However, a surgery should be planned to prevent or minimize a postsurgical memory decline especially in resection of a dominant temporal lobe. Cortisectomy is a resection of localized epileptic focus in patients with neocortical epilepsy such as frontal, parietal, occipital, and lateral temporal lobe epilepsies. Most of neocortical epilepsy patients need an intracranial electrode implantation for determination of resection margin and a brain stimulation on intracranial electrodes for functional mapping. For a successful cortisectomy, an epilepsy surgery team should have a good amount of knowledge and experiences in intracranial EEG monitoring for intractable epilepsy patients. It is very important to place the intracranial electrodes at a brain region where epileptic focus is located because a wrong placement of intracranial electrodes results in failure of surgery. The surgical principles of functional hemispherectomy (FH) aim at disconnecting the hemisphere while leaving as much of the ipsilateral brain as possible intracranially; it has been characterized as anatomically subtotal but physiologically complete hemispherectomy. The original technique consists of a large central tissue removal, complete callosotomy, frontal and parieto-occipital disconnection, temporal lobectomy and insular corticectomy. The candidates of FH are drug refractory partial epilepsy patients who have unilateral epileptic focus and severe brain damage in ipsilateral hemisphere with loss of finger movements of contralateral hand. Corpus callosotomy is a surgical technique severing the corpus callosum so that communication between the cerebral hemispheres is interrupted. In contrast with lobectomy, corpus callosotomy does not involve removing any brain tissue. Instead, it usually involves cutting the front two-thirds of this bundle (anterior callosotomy). Sometimes the other one-third is cut later (complete callosotomy). Corpus callosotomy is most effective for atonic seizures ("drop attacks"), less effective for tonic-clonic seizures and tonic seizures. Additionally, multiple subpial transection and neuro-stimulation techniques are described.
Figures and Tables
 | Figure 1Anterior temporal lobectomy with amygdalohippocampectomy. 45 year-old woman had complex partial seizures arising from right mesial temporal region. She has been seizure free since her right anterior temporal lobe and amygdala/hippocampus were resected. The MRI images were obtained from Samsung Medical Center. |
 | Figure 2Focal cortisectomy. Fourteen year-old girl had suffered from intractable complex partial seizures arising from left parietal lobe. She has been seizure free since her left parietal cortisectomy (arrow). The MRI images were obtained from Samsung Medical Center. |
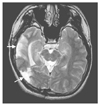 | Figure 3Multilobar resection. Twenty-six year-old man has suffered from complex partial seizures and nocturnal generalized tonic clonic seizures since his age 14. His seizures arose from right temporal and occipital lobes independently. The patient has been seizure free after those two regions were resected (arrows). The MRI images were obtained from Samsung Medical Center. |
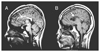 | Figure 4Corpus callosotomy. Thirteen year-old girl (A) and 15 year-old body (B) have suffered from frequent atonic seizures. After anterior corpus callosotomy, seizures frequency decreased to less than 10% of presurgical level in both patients. But the seizure frequency of patient-B increased back to a presurgical state several months after the surgery. The MRI images were obtained from Samsung Medical Center. |
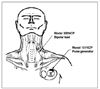 | Figure 5Placement of Pulse Generator and Bipolar Lead for Vagus Nerve Stimulation. The Pulse Generator is usually implanted just below the clavicle in a subcutaneous pocket in the left upper chest. Suggested placement for the Bipolar Lead is the area of the left vagus nerve just above the clavicle, with the Bipolar Lead subcutaneously tunneled between the stimulation site in the neck and the pocket formed in the upper chest. This picture was obtained from Cyberonics website. |
 | Figure 6Deep brain stimulation. Electrodes for deep brain stimulation (two quadri-pole electrodes in dotted circle) are placed in a subthalamic nucleus in a 25 year-old patient with intractable partial epilepsy originated from bilateral parietal lobes. This X-ray image was obtained from Samsung Medical Center. |
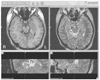 | Figure 7Dose planning for Gamma Knife radiosurgery. The right hippocampus and parahippocampal gyrus are selected for a radiosurgery by Gamma Knife in a 16 year-old girl who had a previous resection of dysembryoplastic neuroepitheleal tumor in right mesial temporal region. The circle is an iso-dose line of 25 Gy. This picture was obtained from Samsung Medical Center. |
References
1. Engel J, Shewmon DA. Engel J, editor. Overview. Who should be considered a surgical candidate? Surgical Treatment of the Epilepsies. 1993. New York: Raven Press;23–43.
2. ILAE Commission Report. The epidemiology of the epilepsies: Future directions. Epilepsia. 1997. 38:614–618.
3. Cascino GD, Trenerry MR, Jack CR Jr, et al. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia. 1995. 36:692–696.

4. Jackson GD, Connelly A, Duncan JS, Grunewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2-relaxometry. Neurology. 1993. 43:1793–1799.

5. Cascino GD. Advances in neuroimaging: surgical localization. Epilepsia. 2001. 42:3–12.
6. Munari C, Bancaud J. Electroclinical symptomatology of partial seizures of orbital frontal origin. Adv Neurol. 1992. 57:257–265.
7. Salanova V, Morris HH, Van Ness P, Kotagal P, Wyllie E, Luders H. Frontal lobe seizures: electroclinical syndromes. Epilepsia. 1995. 36:16–24.

8. Berkovic SF, Rowe CC. Disksic M, Reba RC, editors. The use of SPECT in focal epilepsy. Radiopharmaceuticals and brain pathology studies with PET and SPECT. 1991. Florida: CRC Press;257–266.

9. Chugani HT, Shewmon DA, Peacock WJ, Shields WD, Mazziotta JC, Phelps ME. Surgical treatment of intractable neonatal-onset seizures: The role of positron emission tomography. Neurology. 1988. 38:1178–1188.

10. Luders H, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988. Suppl 2. S56–S65.

11. Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behav Brain Sci. 1983. 6:190–206.

12. Villemure JG, Mascott CR. Periinsular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995. 37:975–981.
13. Spencer DD, Spencer SS. Corpus callosotomy in the treatment of medically intractable secondarily generalized seizures in children. Cleve Clin J Med. 1988. 56:Suppl. 69–77.
14. Spencer SS, Spencer DD, Williamson PD, Sass K, Novelly RA, Mattson RH. Corpus callosotomy for epilepsy. I. Seizure effects. Neurology. 1988. 38:19–24.

15. Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005. 46:Suppl 1. 30–31.

16. Sass KJ, Spencer DD, Spencer SS, Novelly RA, Williamson PD, Mattson RH. Corpus callosotomy for epilepsy. II. Neurologic and neuropsychological outcome. Neurology. 1988. 38:24–28.

17. Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001. 42:67–71.

18. Morrell F, Whisler WW, Bleck T. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989. 70:231–239.

19. Spencer SS, Schramm J, Wyler A, et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002. 43:141–145.

20. Orbach D, Romanelli P, Devinsky O, Doyle W. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001. 42:1316–1319.

21. Hufnagel A, Zentner J, Fernandez G, Wolf HK, Schramm J, Elger CE. Multiple subpial transection for control of epileptic seizures: effectiveness and safety. Epilepsia. 1997. 38:678–688.

23. The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995. 45:224–230.
24. DeGiorgio C, Heck C, Bunch S, et al. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology. 2005. 65:317–319.

25. Uthman BM, Reichl AM, Dean JC, Eisenschenk S, Gilmore R, Wilder BJ, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004. 63:1124–1126.

26. Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002. 52:556–565.

27. Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002. 06. 43(6):603–608.

28. Baker KB, Montgomery EB Jr, Rezai AR, Burgess R, Luders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiological and therapeutic implications. Mov Disord. 2002. 17:969–983.

29. Regis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Pendl G, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004. 45:504–515.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download