Abstract
Epilepsy is a chronic neurological disorder manifesting recurrent unprovoked epileptic seizures. About 20~30% of epilepsy patients are resistant to antiepileptic medications. These patients suffer from high risk of physical injury, unemployment, marital problem, and psychological stress. Epilepsy surgery is the firstly recommended treatment modality for the patients with medically intractable epilepsy. Presurgical evaluation is the most important process for performing epilepsy surgery. The ultimate goal of the presurgical evaluation in patients with medically refractory partial seizures is the localization of the epileptogenic zone and the resection of which is also both necessary and sufficient to render the patient seizure-free. The localization of the epileptogenic zone derives from a hierarchical synthesis of localizing data independently obtained from clinical, electrographic, neuroimaging, and neuropsychological examination. In addition, closely related to the goal of localizing the epileptogenic zone is the significant need for anticipating the risks of functional deficits that could derive from the surgical resection. Mesial temporal lobe epilepsy (TLE) is the best candidate for epilepsy surgery. Anterior temporal lobectomy with amygdalohippocampectomy is a surgical treatment method for mesial TLE and its seizure-free rate (SFR) is 60~90%, whereas one-year SFR of antiepileptic drug treatment for mesial TLE is 10~20%. Cortisectomy is a surgical method for extratemporal epilepsy and its SFR is about 40~70%. Corpus callosotomy is a partial or complete division of corpus callosum for preventing seizure propagations between right and left hemispheres and is effective for controlling atonic seizures. The variation of postsurgical seizure outcomes is related to the qualities of epilepsy surgery program, presurgical evaluation and surgical techniques. For the good surgical outcome, the epilepsy surgery program should include neurologist, neurosurgeon, neuropsychologist, neuro-radiologist and neuro-nuclear medicine specialist for a comprehensive team approach.
Figures and Tables
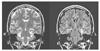 | Figure 1
Left hippocampal sclerosis. T2 weighted (left) and fluid attenuated inversion recovery (FLAIR, right) MR images show decreased volume and increased signal in left hippocampus (arrow) in a patient with left mesial temporal lobe epilepsy. MRI images were obtained from Samsung Medical Center. |
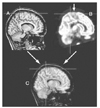 | Figure 2
MRI-PET co-registration. A 7 year-old girl has suffered from frequent supplementary motor area seizures occurring several times per a night. Her brain MRI (A) showed no abnormality whereas brain FDG-PET (B) revealed a definite hypometabolism on left medical frontal region (arrow). Fused image (C) of MRI and FDG-PET could localize the hypometabolic zone on the patient's MRI. MRI and FDG-PET images were obtained from Samsung Medical Center. |
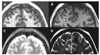 | Figure 3
Transmantle dysplasia right frontal-axial T2 FSE (D) and axial magnetization prepared rapid gradient echo (B), a thin section volumetric T1-weighted sequence (right) obtained with a 3T PA MRI showing a subtle right frontal cone-shaped region of increased T2 signal that begins at the ventricular margin and extends to the depth of a sulcus (see arrowheads in D). The lesion corresponds to a more subtle region of decreased T1 signal (arrowheads, B). On close scrutiny of the overlying cortex, increased T2 signal (arrows, D) and increased T1 signal (arrows, B) with blurring of the gray-white junction is identified. Previous high resolution 1.5T MRI obtained with a regular head coil did not show the lesion (A, T1-weighted image, C, T2-weighted image). Due to different imaging protocols, the two images are angled slightly differently and have different slice thickness (1.5T images courtesy Dr. P. Due Tⓒ™nnessen, Dept. of Radiology, Rikshospitalet Olso, Norway). This figure was quoted on the permission of Dr. P. Ellen Grant. |
 | Figure 4
24-hour video-EEG monitoring unit. In the video-EEG monitoring room, patients' EEG and behavior are being recorded and monitored by Vanguard EEG system and trained EEG technicians. If a patient starts having a seizure, a technician should run into a monitoring room and perform a seizure interview, and may inject a radiotracer for ictal SPECT study. This picture was obtained from Epilepsy Monitoring Unit in Samsung Medical Center. |
 | Figure 5
Ictal scalp EEG recorded during clinical seizures shows rhythmic discharges (arrow) on left temporal lobe with many muscle artifacts (arrow head). The EEG montage in left column indicates left hemisphere by odd numbers whereas right hemisphere by even numbers. This patient has a left hippocampal sclerosis on her brain MRI. She has been seizure free after her anterior temporal lobe and amygdala/hippocampus were resected. The EEG data were obtained from Samsung Medical Center. |
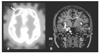 | Figure 6
Ictal SPECT and SISCOM. Ictal SPECT (A) shows increased cerebral blood flow on right anterior frontal region (arrow). SISCOM (B) shows very well localized ictal hyperperfusion (arrow) on right hippocampus and insular cortex in a patient with right mesial temporal lobe epilepsy. Ictal SPECT and SISCOM images were obtained from Samsung Medical Center. |
 | Figure 7
Intracranial electrodes. (A) is a 1×8 subdural strip electrode, (B) is a 4×8 subdural grid electrode and (C) is a 1×8 depth electrode. Subdural electrodes are placed on cortical surface whereas a depth electrode is placed on deeper brain structures such as bilateral hippocampi and interhemispheric medial frontal regions by piercing cerebral cortex and white matter with either stereotactic frames or with a frameless stereotactic technique. |
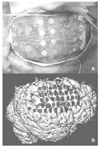 | Figure 8
Subdural grid electrode insertion. (A) shows subdural grid electrodes (4×8) placed on right frontoparietal cortex. (B) demonstrates the location of subdural grid (4×8) and strip (1×8) electrodes on the patient's 3D brain by a CT-MRI co-registration technique. The images were obtained from Samsung Medical Center. |
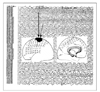 | Figure 9
Intracranial cortical EEG. This patient is a 7 year-old girl who had suffered from frequent bilateral tonic seizures with preserved consciousness. Well localized EEG seizure discharges (low amplitude rhythmic fast activities) were recorded during clinical seizures on left surperior frontoparietal regions of subdural grid electrodes. Intracranial EEG recording and a functional map by electrical cortical stimulation are necessary for determining a resection margin of neocortical epilepsy. The intracranial EEG data were obtained from Samsung Medical Center. |
 | Figure 10Decision making cascade for intractable epilepsy patients
mTLE: mesial temporal lobe epilepsy, Neo.: neocortical, Gen.: generalized, HS: hippocampal sclerosis, Uni.: unilateral, Bi: bilateral, FDG-PET: 18F-fluorodeoxy glucose positron emission tomography, hypom: hypometabolism, electr: electrode, ipsi: ipsilateral to epileptic focus, contra: contralateral to epileptic focus, MST: multiple subpial transection, ECoG: intraoperative electro-corticography, PKC: paroxysmal kinesogenic choreoathetosis, Ipsi ≫ contra: Ipsilateral hippocampal seizure origin is much more frequent than contralateral origin. Ipsi ≈ contra: Ipsilateral hippocampal seizure frequency is similar to contralateral seizure frequency. TIA: transient ischemic attack, ATL & AH: anterior temporal lobectomy & amygdalohippocampectomy.
|
Table 2
Contents of presurgical evaluation

Performed invariably: almost always obtained prior to epilepsy surgery. Performed variably: available at most epilepsy centers, used in selected candidates. Performed in selected centers: not widely available. MRI: magnetic resonance imaging, PET: positron emission tomography, SPECT: single photon emission computed tomography. SISCOM: subtraction ictal SPECT co-registered to MRI, MRS: magnetic resonance spectroscopy, MEG: magnetoencephalography, MRI special study: volumetry, 3-D rendering, gyroscan, etc.
Acknowledgement
This work was supported by a grant(03-PJ1-PG3-21300-0033) of the Good Health R&D Project, Ministry of Health & Welfare, Republic of Korea.
References
1. Penfield W, Flanigin H. Surgical therapy of temporal lobe seizures. Arch Neurol Psychiatry. 1950. 64:491–500.

2. Bailey P, Gobbs FA. The surgical treatment of psychomotor epilepsy. J Am Med Assoc. 1951. 145:365–370.

3. Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, Enos B, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. 2003. 44:741–751.

4. Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Baulac M, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998. 51:1256–1262.

5. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345:311–318.

6. Kuzniecky R, Jackson G. Neuroimaging in epilepsy: magnetic resonance in epilepsy. 1995. New York: Raven Press;27–48.
7. Jack CR Jr, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formations. Radiology. 1990. 175:423–429.

8. Jack C, Rydberg C, Kreacker K, et al. Mesial temporal sclerosis: diagnosis with FLAIR versus spin-echo MR imaging. Radiology. 1996. 199:367–373.

9. Jackson GD, Berkovic SF, Duncan JS, et al. Optimizing the diagnosis of hippocampal sclerosis using magnetic resonance imaging. AJNR Am J Neuroradiol. 1993. 14:753–762.
10. Kuzniecky R, Cascino GD, Palmini A, et al. Engel J, editor. Structural imaging. Surgical treatment of the epilepsies. 1993. 2nd ed. New York: Raven Press;197–200.
11. Gadian DG. Nuclear magnetic resonance and its applications to living systems. 1982. New York: Oxford University Press.
12. Cascino GD, Jack CR Jr, Hirschorn KA, Sharbrough FW. Identification of the epileptic focus: magnetic resonance imaging. Epilepsy Res Suppl. 1992. 5:95–100.
13. Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Grant PE, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005. 65:1026–1031.

14. Lagerlund TD, Cascino GD, Cicora KM, Sharbrough FW. Long-term electroencephalographic monitoring for diagnosis and management of seizures. Mayo Clin Proc. 1996. 71:1000–1006.

16. Spencer SS, Theodore WH, Berkovic SF. Clnical applications: MRI, SPECT, and PET. Magn Reson Imaging. 1995. 13:1119–1124.
17. Lee HW, Hong SB, Tae WS. Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000. 123:2150–2150.

18. O'Brien TJ, So EL, Mullan BP, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000. 55:1668.
19. Choi JY, Kim SJ, Hong SB, et al. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003. 30:581–587.

20. Hong SB, Kim KW, Seo DW, Kim SE, Na DG, Byun HS. Contralateral EEG slowing and amobarbital distribution in Wada test: an intracarotid SPECT study. Epilepsia. 2000. 41:207–212.

21. Hong SB, Han HJ, Roh SY, Seo DW, Kim SE, Kim MH. Hypometabolism and interictal spikes during positron emission tomography scanning in temporal lobe epilepsy. Eur Neurol. 2002. 48:65–70.

22. Behrens E, Zentner J, van Roost D, Hufnagel A, Elger CE, Schramm J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochir (Wien). 1994. 128:84–87.

23. Wass CT, Grady RE, Fessler AJ, et al. The effects of remifentanil on epileptiform discharges during intraoperative electrocorticography in patients undergoing epilepsy surgery. Epilepsia. 2001. 42:1340–1344.

24. Fiol ME, Gates JR, Torres F, Maxwell RE. The prognostic value of residual spikes in the postexcision electrocorticogram after temporal lobectomy. Neurology. 1991. 41:512–516.

25. Engel J Jr, Wiebe S, French J, Sperling M, Williamson P, Gumnit R, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003. 60:538–547.

26. Daniel RT, Joseph TP, Gnanamuthu C, Chandy MJ. Hemispherotomy for paediatric hemispheric epilepsy. Stereotact Funct Neurosurg. 2001. 77:219–222.

27. Pulsifer MB, Brandt J, Salorio CF, Vining EP, Carson BS, Freeman JM. The cognitive outcome of hemispherectomy in 71 children. Epilepsia. 2004. 45:243–254.

28. Spencer SS, Schramm J, Wyler A, O'Connor M, Orbach D, Krauss G, et al. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002. 43:141–145.

29. Orbach D, Romanelli P, Devinsky O, Doyle W. Late seizure recurrence after multiple subpial transections. Epilepsia. 2001. 42:1130–1133.

30. Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005. 128:1188–1198.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download