Abstract
Objective
We conducted a pooled analysis of published studies to compare the performance of human papillomavirus (HPV) testing and cytology in detecting residual or recurrent diseases after treatment for cervical intraepithelial neoplasia grade 2 or 3 (CIN 2/3).
Methods
Source articles presenting data on posttreatment HPV testing were identified from the National Library of Medicine (PubMed) database. We included 5,319 cases from 33 articles published between 1996 and 2013.
Results
The pooled sensitivity of high-risk HPV testing (0.92; 95% confidence interval [CI], 0.90 to 0.94) for detecting posttreatment CIN 2 or worse (CIN 2+) was much higher than that of cytology (0.76; 95% CI, 0.71 to 0.80). Co-testing of HPV testing and cytology maximized the sensitivity (0.93; 95% CI, 0.87 to 0.96), while HPV genotyping (detection of the same genotype between pre- and posttreatments) did not improve the sensitivity (0.89; 95% CI, 0.82 to 0.94) compared with high-risk HPV testing alone. The specificity of high-risk HPV testing (0.83; 95% CI, 0.82 to 0.84) was similar to that of cytology (0.85; 95% CI, 0.84 to 0.87) and HPV genotyping (0.83; 95% CI, 0.81 to 0.85), while co-testing had reduced specificity (0.76; 95% CI, 0.75 to 0.78). For women with positive surgical margins, high-risk HPV testing provided remarkable risk discrimination between test-positives and test-negatives (absolute risk of residual CIN 2+ 74.4% [95% CI, 64.0 to 82.6] vs. 0.8% [95% CI, 0.15 to 4.6]; p<0.001).
Conclusion
Our findings recommend the addition of high-risk HPV testing, either alone or in conjunction with cytology, to posttreatment surveillance strategies. HPV testing can identify populations at greatest risk of posttreatment CIN 2+ lesions, especially among women with positive section margins.
Go to : 
References
1. Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006; 24(Suppl 3):S3/78–89.

2. Chan BK, Melnikow J, Slee CA, Arellanes R, Sawaya GF. Posttreatment human papillomavirus testing for recurrent cervical intraepithelial neoplasia: a systematic review. Am J Obstet Gynecol. 2009; 200:422. e1–9.

3. Kocken M, Uijterwaal MH, de Vries AL, Berkhof J, Ket JC, Helmerhorst TJ, et al. High-risk human papillomavirus testing versus cytology in predicting posttreatment disease in women treated for high-grade cervical disease: a systematic review and meta-analysis. Gynecol Oncol. 2012; 125:500–7.

4. Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006; 118:2048–55.

5. Takeda T, Wong TF, Adachi T, Ito K, Uehara S, Kanaoka Y, et al. Japan Society of Obstetrics and Gynecolo-gyJapan Association of Obstetricians and Gynecologists. Guidelines for office gynecology in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2011 edition. J Obstet Gynaecol Res. 2012; 38:615–31.

6. IKNL. (integraal kankercentrum Nederland) Oncoline. National Guideline “Cervical Intraepithelial Neoplasia” 2004 [Internet]. Utrecht, ND: Comprehensive Cancer Centre the Netherlands;c2015. [cited 2015 Nov 4]. Available from:. http://www.oncoline.nl/index.php?pagina=/richtlijn/item/pagina.php&richt-lijn_id=220.
7. NHS (National Health Service) Cancer Screening Programmes. Colposcopy and programme management: guideline for the NHS cervical cancer screening progamme: second edition [Internet]. Sheffield, UK: NHS Cancer Screening Programmes;2010. [cited 2015 Nov 4]. Available from:. http://www.cancerscreening.nhs.uk/cervical/publications/nhscsp20.html.
8. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013; 121:829–46.
9. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014; 383:524–32.
10. Bollen LJ, Tjong-A-Hung SP, van der Velden J, Mol BW, ten Kate FW, ter Schegget J, et al. Prediction of recurrent and residual cervical dysplasia by human papillomavirus detection among patients with abnormal cytology. Gynecol Oncol. 1999; 72:199–201.

11. Hernádi Z, Szoke K, Sápy T, Krasznai ZT, Soós G, Veress G, et al. Role of human papillomavirus (HPV) testing in the follow-up of patients after treatment for cervical precancerous lesions. Eur J Obstet Gynecol Reprod Biol. 2005; 118:229–34.

12. Almog B, Gamzu R, Kuperminc MJ, Levin I, Fainaru O, Niv J, et al. Human papilloma virus testing in patient follow-up post cone biopsy due to high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2003; 88:345–50.

13. Elfgren K, Bistoletti P, Dillner L, Walboomers JM, Meijer CJ, Dillner J. Conization for cervical intraepithelial neoplasia is followed by disappearance of human papillomavirus deoxyribonucleic acid and a decline in serum and cervical mucus antibodies against human papillomavirus antigens. Am J Obstet Gynecol 1996;174:937–42.
14. Distéfano AL, Picconi MA, Alonio LV, Dalbert D, Mural J, Bartt O, et al. Persistence of human papillomavirus DNA in cervical lesions after treatment with diathermic large loop excision. Infect Dis Obstet Gynecol. 1998; 6:214–9.

15. Bekkers RL, Melchers WJ, Bakkers JM, Hanselaar AG, Quint WG, Boonstra H, et al. The role of geno-type-specific human papillomavirus detection in diagnosing residual cervical intraepithelial neoplasia. Int J Cancer. 2002; 102:148–51.

16. Kreimer AR, Guido RS, Solomon D, Schiffman M, Wacholder S, Jeronimo J, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006; 15:908–14.

17. Brismar S, Johansson B, Borjesson M, Arbyn M, Andersson S. Follow-up after treatment of cervical intraepithelial neoplasia by human papillomavirus genotyping. Am J Obstet Gynecol. 2009; 201:17. e1–8.

18. Kang WD, Oh MJ, Kim SM, Nam JH, Park CS, Choi HS. Significance of human papillomavirus genotyping with high-grade cervical intraepithelial neoplasia treated by a loop electrosurgical excision procedure. Am J Obstet Gynecol. 2010; 203:72. e1–6.

19. Heymans J, Benoy IH, Poppe W, Depuydt CE. Type-specific HPV geno-typing improves detection of recurrent high-grade cervical neoplasia after conisation. Int J Cancer. 2011; 129:903–9.

20. Valasoulis G, Koliopoulos G, Founta C, Kyrgiou M, Tsoumpou I, Valari O, et al. Alterations in human papillomavirus-related biomarkers after treatment of cervical intraepithelial neoplasia. Gynecol Oncol 2011;121:43–8.
21. Söderlund-Strand A, Kjellberg L, Dillner J. Human papillomavirus type-specific persistence and recurrence after treatment for cervical dysplasia. J Med Virol. 2014; 86:634–41.

22. Chua KL, Hjerpe A. Human papillomavirus analysis as a prognostic marker following conization of the cervix uteri. Gynecol Oncol. 1997; 66:108–13.

23. Jain S, Tseng CJ, Horng SG, Soong YK, Pao CC. Negative predictive value of human papillomavirus test following conization of the cervix uteri. Gynecol Oncol. 2001; 82:177–80.

24. Bodner K, Bodner-Adler B, Wierrani F, Kimberger O, Denk C, Grünberger W. Is therapeutic conization sufficient to eliminate a high-risk HPV infection of the uterine cervix? A clinicopathological analysis. Anticancer Res. 2002; 22(6B):3733–6.
25. Nagai N, Mukai K, Oshita T, Shiroyama Y, Ohama K. Human papillomavirus DNA status after loop excision for cervical intraepithelial neoplasia grade III – A prospective study. Int J Mol Med. 2004; 13:589–93.

26. Verguts J, Bronselaer B, Donders G, Arbyn M, Van Eldere J, Drijkoningen M, et al. Prediction of recurrence after treatment for high-grade cervical intraepithelial neoplasia: the role of human papillomavirus testing and age at conisation. BJOG. 2006; 113:1303–7.

27. Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007; 17:1271–7.

28. Gallwas J, Ditsch N, Hillemanns P, Friese K, Thaler C, Dannecker C. The significance of HPV in the follow-up period after treatment for CIN. Eur J Gynaecol Oncol. 2010; 31:27–30.
29. Torné A, Fusté P, Rodríguez-Carunchio L, Alonso I, del Pino M, Nonell R, et al. Intraoperative post-coni-sation human papillomavirus testing for early detection of treatment failure in patients with cervical intraepithelial neoplasia: a pilot study. BJOG. 2013; 120:392–9.

30. Ribaldone R, Boldorini R, Capuano A, Arrigoni S, Di Oto A, Surico N. Role of HPV testing in the follow-up of women treated for cervical dysplasia. Arch Gynecol Obstet. 2010; 282:193–7.

31. Leguevaque P, Motton S, Decharme A, Soulé-Tholy M, Escourrou G, Hoff J. Predictors of recurrence in high-grade cervical lesions and a plan of management. Eur J Surg Oncol. 2010; 36:1073–9.

32. Ryu A, Nam K, Kwak J, Kim J, Jeon S. Early human papillomavirus testing predicts residual/recurrent disease after LEEP. J Gynecol Oncol. 2012; 23:217–25.

33. Fallani MG, Penna C, Marchionni M, Bussani C, Pieralli A, Andersson KL, et al. Prognostic significance of high-risk HPV persistence after laser CO2 conization for high-grade CIN: a prospective clinical study. Eur J Gynaecol Oncol. 2008; 29:378–82.
34. Nagai Y, Maehama T, Asato T, Kanazawa K. Persistence of human papillomavirus infection after therapeutic conization for CIN 3: is it an alarm for disease recurrence? Gynecol Oncol. 2000; 79:294–9.
35. Nobbenhuis MA, Meijer CJ, van den Brule AJ, Rozendaal L, Voorhorst FJ, Risse EK, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001; 84:796–801.

36. Zielinski GD, Rozendaal L, Voorhorst FJ, Berkhof J, Snijders PJ, Risse EJ, et al. HPV testing can reduce the number of follow-up visits in women treated for cervical intraepithelial neoplasia grade 3. Gynecol Oncol. 2003; 91:67–73.

37. Cecchini S, Carozzi F, Confortini M, Zappa M, Ciatto S. Persistent human papilloma virus infection as an indicator of risk of recurrence of high-grade cervical intraepithelial neoplasia treated by the loop electrosurgical excision procedure. Tumori. 2004; 90:225–8.

38. Sarian LO, Derchain SF, Andrade LA, Tambascia J, Morais SS, Syrjänen KJ. HPV DNA test and Pap smear in detection of residual and recurrent disease following loop electrosurgical excision procedure of high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2004; 94:181–6.

39. Kitchener HC, Walker PG, Nelson L, Hadwin R, Patnick J, Anthony GB, et al. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG. 2008; 115:1001–7.

40. Smart OC, Sykes P, Macnab H, Jennings L. Testing for high risk human papilloma virus in the initial follow-up of women treated for high-grade squamous intraepithelial lesions. Aust N Z J Obstet Gynaecol. 2010; 50:164–7.

41. Bar-Am A, Gamzu R, Levin I, Fainaru O, Niv J, Almog B. Follow-up by combined cytology and human papillomavirus testing for patients post-cone biopsy: results of a long-term follow-up. Gynecol Oncol. 2003; 91:149–53.

Go to : 
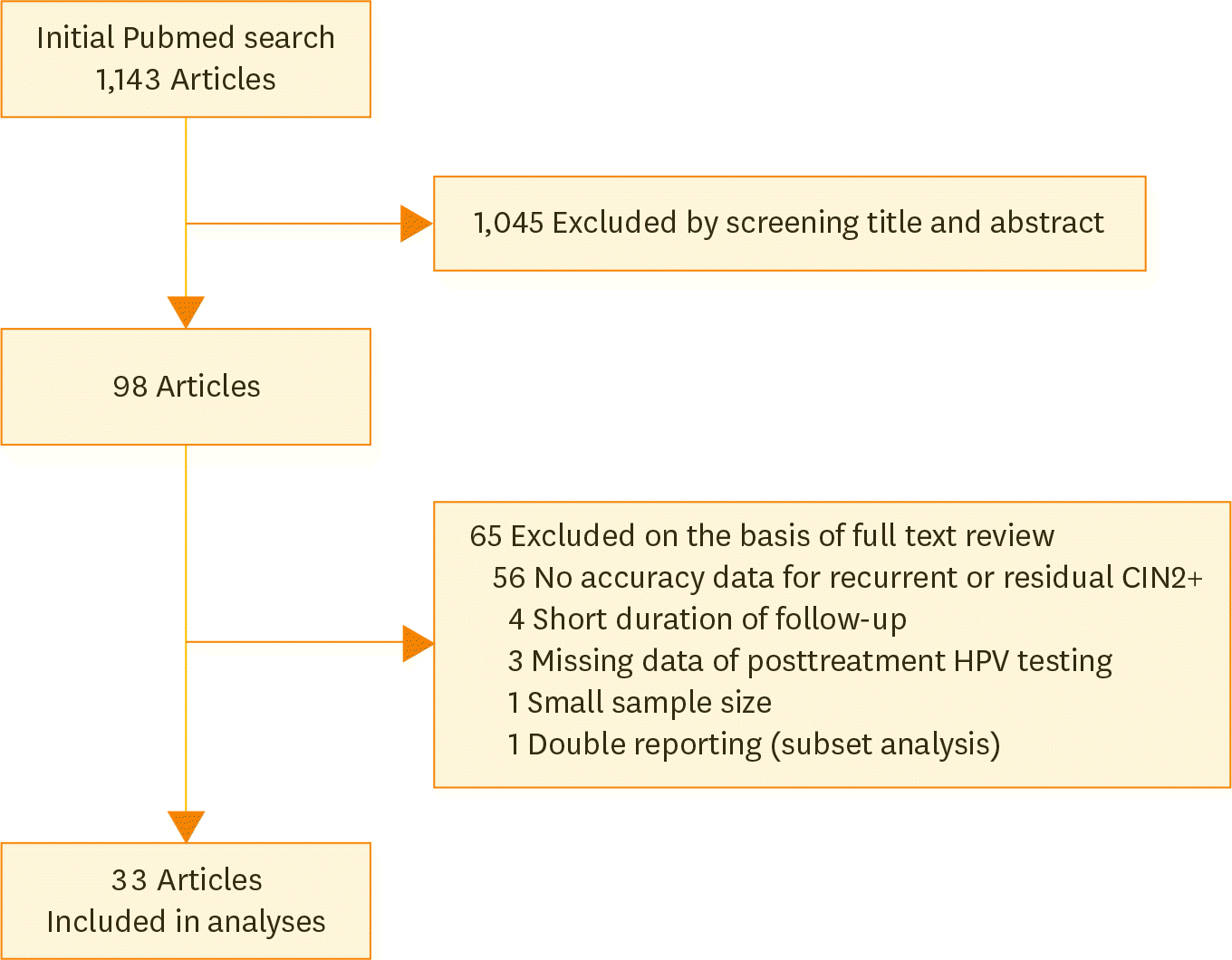 | Fig. 1.Literature search. Initial search through PubMed identified 1,143 articles for our systematic review. After reviews of titles, abstracts and full texts, 33 eligible articles were included in our analysis [10–42]. CIN 2, cervical intraepithelial neoplasia grade 2; HPV, human papillomavirus. |
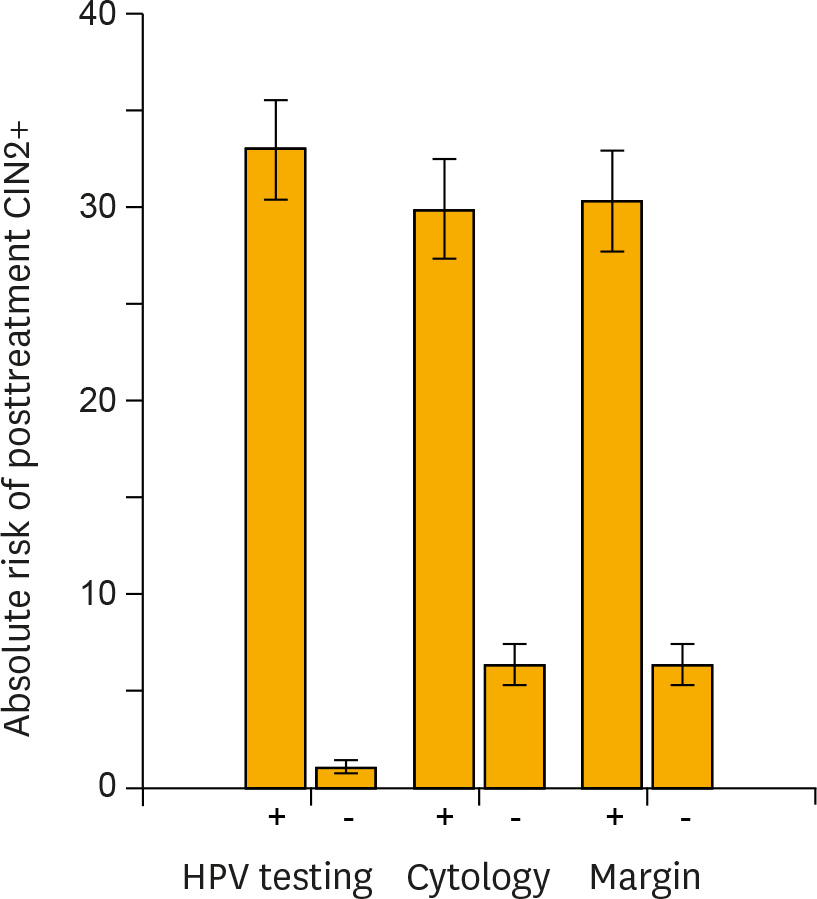 | Fig. 2.Risk stratification of posttreatment cervical intraepithelial neoplasia grade 2+ (CIN 2+) provided by carcinogenic human papillomavirus (HPV) testing, cytology, and surgical margin histology. In each test method of high-risk HPV testing, cytology (atypical squamous cells of undetermined significance+) or surgical margin histology, the absolute risks of having recurrent or residual CIN 2+ lesions (■) and 95% confidence intervals (error bars) were calculated for women testing positive or negative for each test. HPV testing provided the greatest risk stratification between test-positive and -negative women. |
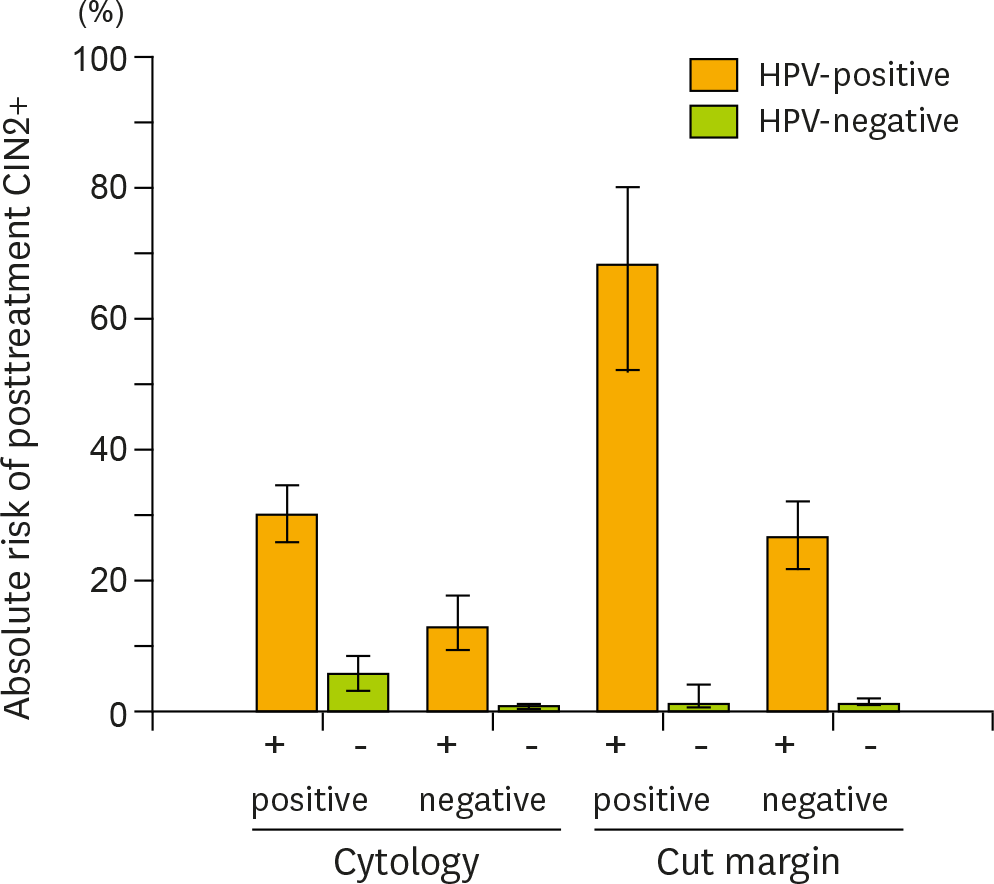 | Fig. 3.Further risk stratification of posttreatment cervical intraepithelial neoplasia grade 2+ (CIN 2+) provided by the addition of carcinogenic human papillomavirus (HPV) testing to cytology and surgical margin histology. Carcinogenic HPV testing provided additional risk stratification for posttreatment CIN 2+ lesions according to cytology results (atypical squamous cells of undetermined significance+) or surgical margin status. The absolute risks of having recurrent or residual CIN 2+ lesions and 95% confidence intervals (error bars) were calculated for HPV-positive (■) and -negative women (■) in each category group. |
Table 1.
Test performance of HPV testing and cytology for detection of posttreatment CIN 2 or worse
| Testing methods | No. of women included in analysis | No. of test-positive women (colposcopy referral rate, %) | Detection of recurrent or residual CIN 2 or worse | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |||
| High-risk HPV test* | 5,322 | 1,119 (21.0) | 0.91 (0.88–0.94) | 0.83 (0.82–0.84) | 0.28 (0.26–0.31) | 0.99 (0.99–1.00) |
| HPV genotyping† | 1,667 | 399 (23.9) | 0.89 (0.82–0.94) | 0.83 (0.81–0.85) | 0.28 (0.24–0.33) | 0.99 (0.98–0.99) |
| Cytology‡ | 3,656 | 813 (22.2) | 0.74 (0.68–0.79) | 0.85 (0.83–0.86) | 0.25 (0.22–0.28) | 0.98 (0.97–0.98) |
| Co-testing§ | 2,287 | 645 (28.2) | 0.92 (0.87–0.96) | 0.76 (0.74–0.78) | 0.20 (0.17–0.23) | 0.99 (0.99–1.00) |
Appendix 1.
Studies included in the pooled analysis of the performance of posttreatment human papillomavirus testing*




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download