Abstract
Objective
Recent investigations have revealed DNA mismatch repair (MMR) gene mutations are closely related with carcinogenesis of endometrial cancer; however the impact of MMR protein expression on prognosis is not determined. Correlations between MMR-related protein expression and clinicopathological factors of endometrial cancers are analyzed in the present study.
Methods
A total of 191 endometrial cancer tissues treated between 1990 and 2007 in our hospital were enrolled. Immunoreactions for MSH2, MLH1, MSH6, and PMS2 on tissue microarray specimens and clinicopathological features were analyzed retrospectively.
Results
Seventy-six cases (40%) had at least one immunohistochemical alteration in MMR proteins (MMR-deficient group). There were statistically significant differences of histology, International Federation of Gynecology and Obstetrics (FIGO) stage, and histological grade between MMR-deficient group and the other cases (MMR-retained group). Response rate of first-line chemotherapy in evaluable cases was slightly higher in MMR-deficient cases (67% vs. 44%, p=0.34). MMR-deficient cases had significantly better progression-free and overall survival (OS) compared with MMR-retained cases. Multivariate analysis revealed MMR status was an independent prognostic factor for OS in endometrial cancers.
Endometrial carcinoma is the most common gynecologic malignancy in Japan with approximately 9,000 to 10,000 cases annually, and the number of patients has been consistently increasing [1,2]. Japanese endometrial cancer cohorts differ from typical American or western European cohort in terms of body mass index (BMI). According to a report from the Organisation for Economic Co-operation and Development (OECD), the prevalence of obesity (BMI >30) varies nearly 10-fold in OECD countries: 4.1% in Japan, 24.8% in United Kingdom, and 36.5% in United States [3]. However, there was no significant difference in frequency of type II endometrial cancers: 13% in Japan, 12% to 17% in America or Europe [4,5,6]. DNA mismatch repair (MMR) gene mutations have been thought to be crucial to tumorigenesis of endometrial cancers [7,8]. Approximately 20% to 30% of endometrial cancers have loss of MMR function; 3% to 5% of these attribute to germline mutation, and the remainder arises due to epigenetic methylation of the MLH1 promoter region causing microsatellite instability (MSI) [9,10,11].
MSI could cause DNA synthetic errors at the region including proto-oncogenes, tumor suppressor genes, and genes responsible for apoptosis such as K-ras, PTEN, TGFβRII, BAX [7]. Subsequently, these secondary genetic alterations may modify the responses to treatment modalities, in addition to causing neoplastic transformation [12,13]. It has been recognized that sensitivity to anticancer drugs can be modulated by MMR status in vitro [14]. Also, modulations of sensitivity to antineoplastic agents have been explained by the status of MMR genes in several human cancers. In colorectal cancer, MSI-high cases had better prognosis than MSI-low or MS stable cases in not only early stage but also advanced stage [15,16,17]. Also, good correlations between MSI status and MMR-related protein expression by immunohistochemistry (IHC) was observed [18,19].
Recent reports suggested that MMR-deficient endometrial cancers are related with unfavorable outcome in women 40 years of age and younger [20,21]. However, the impact of MMR status on prognoses of endometrial cancers has not been determined. Some reports included non-endometrioid cancers, and concluded that MMR-deficient endometrial cancers had better prognoses than MMR-retained cases [22]. Other reports analyzing endometrioid histology only showed no difference in survival outcomes [23,24]. Although a meta-analysis including all histologic subtypes suggested that there was no impact of MMR status upon prognoses in endometrial cancers [25], the conclusions were not based upon multivariate analyses.
In this study, we aimed to investigate correlations between MMR-related protein expression and clinicopathological features in endometrial cancer using IHC.
After obtaining Institutional Review Board approval, 191 surgically resected endometrial cancer tissues between 1990 and 2007 in our institution were enrolled and tissue microarray (TMA) was prepared for evaluation. 1.5-mm cores were punched from formalin-fixed paraffin-embedded donor blocks, and inserted into a recipient block. All specimens were cutoff 4-µm-thick sections. Duplicate cores were obtained from each sample. Immunoreactions for MSH2, MLH1, MSH6, and PMS2 were examined on TMA specimens. Mouse monoclonal antibody for MSH2 (D219-1129, dilution 1:80, BD Pharmingen, San Jose, CA, USA), MLH1 (G168-15, dilution 1:80, BD Pharmingen), MSH6 (44, dilution 1:200, BD Transduction Labs, San Jose, CA, USA), and PMS2 (A16-4, dilution 1:50, BD Pharmingen) were used.
Sections were deparaffinized and boiled in an autoclave at 121℃ for 15 minutes in 0.01 mol/L citrate buffer (pH 6.0) for detection of MSH2, MLH1, and PMS2 or warmed in a hot water at 80℃ for an hour in 0.01 mol/L citrate buffer (pH 6.0) for detection of MSH6, and then allowed to cool at room temperature. Endogenous peroxidase was blocked using 0.3% hydrogen peroxidase added to methanol. The slides were incubated at 4℃ overnight with primary antibodies and then reacted with a dextran polymer reagent combined with secondary antibodies and peroxidase (Dako Envision+System-HRP Labelled Polymer, Dako North America Inc., Camarillo, CA, USA) for an hour at room temperature. Specific antigenantibody reactions were visualized with 0.2% diaminobenzidine tetrahydrochloride and hydrogen peroxidase, and counterstaining was performed using Mayer hematoxylin. Stromal cells and lymphocytes in the sections were served as built-in positive controls. As negative controls, sections without the primary antibodies were used.
The immunostaining was evaluated in areas with well-preserved tissue morphology and without necrosis or artifacts. For all of the four markers detection, a nuclear immunoreaction was taken into account for evaluation. The lesions were considered as positive for each marker if tumor cells in the interest area showed immunoreactive intensity stronger than or equal to positive controls. The lesions were considered as negative for each marker if tumor cells showed complete loss of immunoreaction. The assignment of immunoreaction was performed independently by two observers (MK and MM), and any discrepancies between the two observers were resolved by conferring over a multiviewer microscope. Cases that at least one of four proteins was judged as negative were assigned to MMR-deficient cases and the remainder cases were assigned to MMR-retained cases.
For the entire period of enrollment of the patients, primary surgery included a simple abdominal hysterectomy, bilateral salpingo-oophorectomy, and peritoneal washing cytology. Lymph node dissection/sampling and/or omentectomy were undergone in the cases that had deep myometrial invasion judged intraoperative inspection, and those that had type II histology by preoperative biopsy. Adjuvant chemotherapy was considered for the patients that had intermediate-high risk factors. Chemotherapy regimen was mainly cyclophosphamide, adriamycin, and cisplatin until 2004, and taxane and platinum combination since 2005. Adjuvant radiotherapy was not usually performed during this period; however, four cases received radiotherapy by physician's choice.
Clinicopathological factors including progression-free survival (PFS) and overall survival (OS) were compared in two groups. Statistical analyses were performed using Stat Mate IV software (ATMS, Tokyo, Japan) and Statview ver. 5 (SAS Institute Japan Ltd., Tokyo, Japan). The t-test and chi-square test were used for comparison of characteristics of two groups. Kaplan-Meier method and log rank test were used for survival analyses. Prognostic significance was analyzed by Cox proportional hazard model using variables as shown below: age, BMI, histology (endometrioid vs. non-endometrioid), grade (grade1/2 vs. grade 3), International Federation of Gynecology and Obstetrics (FIGO) stage (I/II vs. III/IV), residual tumor (no vs. yes), and MMR status (deficient vs. retained). The differences at p-value less than 0.05 were considered to be statistically significant.
Among 191 cases evaluated, frequencies of MMR-related protein loss were observed in 53 cases (28%) by MLH1, 25 cases (15%) by MSH2, 27 cases (14%) by MSH6, and 37 cases (19%) by PMS2, respectively. A total of 76 cases (40%) were judged as MMR-deficient status. Patient characteristics according to MMR status was shown in Table 1. Loss of expression status about MMR-related proteins was shown in Table 2. There were statistically significant differences of FIGO stage, histology, and grade between two groups. On the other hand, there were no significant differences in age, BMI, frequencies of residual tumor, lymph node dissection, synchronous ovarian cancer, and metachronous colon/gastric/ovarian cancer.
A total of 75 cases received adjuvant chemotherapy, and assessment using Response Evaluation Criteria in Solid Tumor was possible in 18 MMR-retained cases and six deficient cases (Table 3). Although there was no significant difference of response rate between two groups (8/18 [44%] vs. 4/6 [67%], p=0.34), two cases that achieved complete response (CR) were MMR-deficient cases.
Five-year PFS was 92% in MMR-deficient patients, and 78% in MMR-retained patients (p=0.013), and 5-year OS was 94% in MMR-deficient patients, and 78% in MMR-retained patients (p=0.009) (Fig. 1). PFS and OS rates were not affected by the amount of MMR protein loss. Five-year PFS rates were 88% in single protein loss, 97% in double protein loss, 92% in triple protein loss, 100% in four protein loss. Five-year OS rates were 92% in single protein loss, 97% in double protein loss, 92% in triple protein loss, and 100% in four protein loss, respectively.
In multivariate analyses, MMR-deficient status was identified as an independent better prognostic factor for OS in endometrial cancers (hazard ratio, 0.24; 95% confidence interval, 0.08 to 0.70; p=0.008) (Table 4).
In this study, frequencies of MMR-related protein loss were 14% to 28% of endometrial cancers and 40% of enrolled cases were judged as loss of MMR expression. Previous reports investigating expression of MMR-related proteins using IHC have shown that approximately 16% to 45% of endometrial cancer had MMR-deficient status [20,21,26]. The frequency of MMR-deficient status as observed in the present study was almost similar to the previous reports, suggesting there was no ethnic difference of frequency in MMR-related endometrial cancers. In current study, 70 of 76 MMR-deficient cases (92%) had endometrioid histology. According to Japanese gynecologic oncology committee, Lynch syndrome-related endometrial cancers in Japan were characterized by early-staged, well-differentiated, endometrioid cancer with favorable outcome [27]. On the other hand, six cases (8%) with non-endometrioid histology consisted of two cases with mucinous carcinoma, two cases with mixed type carcinoma, one case with serous carcinoma, and one case with undifferentiated carcinoma. Previous reports have documented that approximately 67% to 94% of MMR-deficient endometrial cancers had endometrioid histology, and the remainders had non-endometrioid histology including serous, clear-cell, undifferentiated carcinoma, and carcinosarcoma [20,21,22,26]. The fraction of type II endometrial carcinoma in current study was also almost the same as previous reports.
Resnick et al. [28] reported that subgroup of patients with non-endometrioid cancer and MMR-deficient had improved survival after adjuvant radiotherapy, suggesting that MMR-deficient status might provide predisposition to be sensitive to adjuvant radiotherapy. Only four cases received adjuvant radiotherapy in current study, and we could not conclude the sensitivity to radiotherapy according to MMR status.
In the present study, MMR-deficient status was identified as a better prognostic marker for OS. A previous meta-analysis showed that the deficiency in MMR was related to worse trends in PFS and OS, although the differences were not significant [25]. Heterogeneity regarding with histology, and adjuvant treatment could possibly lead to other results. Actually, a meta-analysis alerted that there was a marked interstudy heterogeneity in the estimates of OS and PFS between studies [25]. Remarkably, the majority of patients with high-to-intermediate risk received postoperative chemotherapy as adjuvant chemotherapy in the present study, which might lead to a significant better OS in the patients with MMR-deficient cases.
The Gynecologic Oncology Group study 122 showed the superiority of PFS by chemotherapy with doxorubicin plus cisplation in patients with stage III-IV endometrial cancers, compared with radiation therapy [29]. In addition, the Japanese Gynecologic Oncology Group study suggested a survival advantage of chemotherapy in the patients with high-to-intermediate risk group (stage IC, >70 years of age, grade 3, stage II, or positive washing cytology with >50% myometrial invasion) [30]. As a result, adjuvant chemotherapy was often used for the endometrial cancer patients with high-to-intermediate risk group in the Japanese Gynecologic Oncology Group [31]. Although there was no significant difference, response rate was higher in MMR-deficient cases compared with MMR-retained patients: 67% vs. 44%. Of note, two cases that achieved CR were MMR-deficient patients. The significance of MMR-related protein expression might be contributed by higher abundance of patients that received adjuvant chemotherapy. Additionally, response to second-line or third-line chemotherapy might be modulated by MMR status. Selection of drugs according to MMR status could possibly increase overall response rates for primary and/or recurrent endometrial cancers.
In conclusion, significant improvement of OS was observed in MMR-deficient cases compared with MMR retained cases. MMR-deficient status was an independent prognostic factor for OS in endometrial cancers. Although further analyses are needed to confirm the results, MMR status could be a key biomarker for predicting response of primary chemotherapy and prognoses in endometrial cancers.
Figures and Tables
Fig. 1
Kaplan-Meier survival curves of all cases according to mismatch repair (MMR) status. (A) Progression-free survival (PFS) curve of MMR-retained cases (dotted line) and MMR-deficient cases (solid line). Five-year PFS was 92% in MMR-deficient patients, and 78% in MMR-retained patients (p=0.013). (B) Overall survival (OS) curves of MMR-retained cases (dotted line) and MMR-deficient cases (solid line). Five-year OS was 94% in MMR-deficient patients, and 78% in MMR-retained patients (p=0.009).

Table 1
Patient characteristics according to MMR-related protein expression

Table 2
Profile of MMR-related protein loss in MMR-deficient endometrial cancers (n=76)
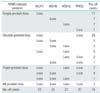
Table 3
Tumor response of adjuvant chemotherapy in evaluable cases

Table 4
Multivariate analysis for progression-free survival and overall survival

ACKNOWLEDGEMENT
I would like to thank Junko Hirata and Ayako Suzuki for their continuing assistance.
References
1. Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2013; 43:328–336.
2. Hirai Y, Banno K, Suzuki M, Ichikawa Y, Udagawa Y, Sugano K, et al. Molecular epidemiological and mutational analysis of DNA mismatch repair (MMR) genes in endometrial cancer patients with HNPCC-associated familial predisposition to cancer. Cancer Sci. 2008; 99:1715–1719.
3. OECDiLibrary. Health at a glance [Internet]. Paris: OECD;2013. cited 2014 Sep 9. Available from: http://dx.doi.org/10.1787/health_glance-2013-en.
4. Japanese Gynecologic Oncology Committee. Annual report of endometrial carcinoma patients in 2006. Acta Obstet Gynaecol Jpn. 2008; 60:1034–1040.
5. Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013; 129:277–284.
6. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013; 31:2607–2618.
7. Karamurzin Y, Rutgers JK. DNA mismatch repair deficiency in endometrial carcinoma. Int J Gynecol Pathol. 2009; 28:239–255.
8. Garg K, Soslow RA. Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma. J Clin Pathol. 2009; 62:679–684.
9. Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998; 95:6870–6875.
10. Boland CR, Koi M, Chang DK, Carethers JM. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer. 2008; 7:41–52.
11. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993; 260:816–819.
12. Hewish M, Lord CJ, Martin SA, Cunningham D, Ashworth A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol. 2010; 7:197–208.
13. Vilar E, Scaltriti M, Balmana J, Saura C, Guzman M, Arribas J, et al. Microsatellite instability due to hMLH1 deficiency is associated with increased cytotoxicity to irinotecan in human colorectal cancer cell lines. Br J Cancer. 2008; 99:1607–1612.
14. Damia G, D'Incalci M. Genetic instability influences drug response in cancer cells. Curr Drug Targets. 2010; 11:1317–1324.
15. Benatti P, Gafa R, Barana D, Marino M, Scarselli A, Pedroni M, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005; 11:8332–8340.
16. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349:247–257.
17. Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996; 56:4881–4886.
18. Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002; 20:1043–1048.
19. Modica I, Soslow RA, Black D, Tornos C, Kauff N, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007; 31:744–751.
20. Garg K, Shih K, Barakat R, Zhou Q, Iasonos A, Soslow RA. Endometrial carcinomas in women aged 40 years and younger: tumors associated with loss of DNA mismatch repair proteins comprise a distinct clinicopathologic subset. Am J Surg Pathol. 2009; 33:1869–1877.
21. Shih KK, Garg K, Levine DA, Kauff ND, Abu-Rustum NR, Soslow RA, et al. Clinicopathologic significance of DNA mismatch repair protein defects and endometrial cancer in women 40 years of age and younger. Gynecol Oncol. 2011; 123:88–94.
22. Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006; 24:1745–1753.
23. Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007; 25:2042–2048.
24. Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000; 89:1758–1764.
25. Diaz-Padilla I, Romero N, Amir E, Matias-Guiu X, Vilar E, Muggia F, et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013; 88:154–167.
26. Grzankowski KS, Shimizu DM, Kimata C, Black M, Terada KY. Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. Gynecol Oncol. 2012; 126:408–412.
27. Report of gynecologic oncology committee. Committee for frequency of hereditary endometrial cancer and its clinicopathology in Japan. Acta Obstet Gynaecol Jpn. 2009; 61:1540–1542.
28. Resnick KE, Frankel WL, Morrison CD, Fowler JM, Copeland LJ, Stephens J, et al. Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer. Gynecol Oncol. 2010; 117:234–238.
29. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:36–44.
30. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008; 108:226–233.
31. Watanabe Y, Kitagawa R, Aoki D, Takeuchi S, Sagae S, Sakuragi N, et al. Practice pattern for postoperative management of endometrial cancer in Japan: a survey of the Japanese Gynecologic Oncology Group. Gynecol Oncol. 2009; 115:456–459.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download