Abstract
Objective
All patients with stage IB1 cervical cancer do not need to undergo parametrectomy. Some low-risk criteria for parametrial involvement (PI) have been proposed based on pathological findings. The aim of this study was to determine pretreatment risk factors for PI in stage IB1 cervical cancer.
Methods
We retrospectively reviewed 115 patients with stage IB1 cervical cancer who underwent radical hysterectomy or radical trachelectomy. Magnetic resonance imaging (MRI) was performed and serum concentrations of squamous cell carcinoma antigen (SCC-Ag) and cancer antigen 125 (CA-125) were determined in all patients before initial treatment. The following pretreatment factors were investigated: histological variant, maximum tumor diameter, tumor volume (volume index), pelvic lymph node enlargement, and serum tumor markers. Logistic regression analysis was used to select the independent risk factors for PI.
Results
Eighteen of the 115 patients (15.7%) were pathologically diagnosed with PI. Multivariate analysis confirmed the following independent risk factors for PI: MRI-based tumor diameter ≥25 mm (odds ratio [OR], 9.9; 95% confidence interval [CI], 2.1 to 48.1), MRI-based volume index ≥5,000 mm3 (OR, 13.3; 95% CI, 1.4 to 125.0), and positive serum tumor markers SCC-Ag ≥1.5 ng/mL or CA-125 ≥35 U/mL (OR, 5.7; 95% CI, 1.3 to 25.1). Of 53 patients with no risk factors for PI, none had PI.
Standard treatment for International Federation of Gynecology and Obstetrics (FIGO) stage IB1 cervical cancer includes radical hysterectomy [12]. Radical trachelectomy is also a treatment option for women who wish to undergo fertility-sparing treatment in accordance with the National Comprehensive Cancer Network clinical guidelines [2]. Both surgical procedures include parametrectomy as part of the whole procedure. The parametrium is rich in vasculature and autonomic nerve fibers; therefore, resection of parametrial tissue needs a longer operating time, and increases the need for blood transfusion and postoperative complications, particularly bladder dysfunction [3]. In addition, parametrectomy may enhance unsatisfactory obstetrical outcomes in radical trachelectomy [4]. According to previous reports, the incidence of parametrial involvement (PI) is not high: <10% (range, 5.4% to 30.6%) in stage IB1 cervical cancer [5678910111213141516] and <4% (range, 0% to 4.5%) in small-sized (<20 mm) cervical cancer [1415161718]. Some patients with stage IB1 cervical cancer may not need to undergo parametrectomy. It has been reported that less radical surgery including conization, simple trachelectomy, or simple hysterectomy leads to satisfactory oncological outcomes in small-volume stage IB1 cervical cancer [192021]. Low-risk group criteria for PI have been proposed. These studies produced excellent prediction models with risk of PI of <2% [11121314182223]. However, these criteria included pathological findings of the resected specimen; namely, they were postoperative risk assessments. Practically, preoperative risk assessment is needed for surgical decision making. We aimed to determine candidates preoperatively who can safely undergo less radical surgery among patients with stage IB1 cervical cancer.
The present study was carried out using data of patients with FIGO stage IB1 cervical cancer for whom tumor diameter/volume were preoperatively confirmed by magnetic resonance imaging (MRI), and radical hysterectomy or radical trachelectomy was performed. Eligible patients underwent resection of bilateral parametrial tissues. A total of 421 patients with invasive cervical cancer were treated in the National Hospital Organization Hokkaido Cancer Center from January 2008 to June 2014 (Table 1). Of 140 patients who had FIGO stage IB1 disease, eight were treated with radiotherapy. One patient refused to receive any treatment. Of 131 patients who underwent surgical treatment, nine underwent conization or simple hysterectomy instead of radical surgery. Two patients received systemic chemotherapy before surgical treatment. Pretreatment images were not obtained in five patients. These patients were excluded from the present study. Finally, we carried out this study with data from 115 patients with stage IB1 cervical cancer. Approval of the Institutional Review Board was obtained from the hospitals' ethics boards.
MRI was performed on all 115 patients before initial treatment. Images were judged by at least two physicians without knowledge of the clinical findings and survival outcomes. The parameters obtained from MRI were tumor size and pelvic lymph node size. Tumor diameter was measured three dimensionally (anteroposterior, craniocaudal, and horizontal diameters) in either T2-weighted images or gadolinium-enhanced T1-weighted images, which show tumor lesions more clearly. Maximal tumor diameter was used for the present analysis.
Volume index was evaluated in T2- or gadolinium-enhanced T1-weighted images, which show tumor lesions clearly. Volume index was defined as the product of the maximum longitudinal diameter (mm) along the uterine axis; the maximum anteroposterior diameter (mm), namely the thickness, on a sagittal section image; and the maximum horizontal diameter (mm) on a horizontal section image. Lymph node enlargement was defined as a minimal diameter >10 mm, as determined radiologically, according to published recommended guidelines [24].
The serum concentration of squamous cell carcinoma antigen (SCC-Ag) was determined by radioimmunoassay. The cut-off value of 1.5 ng/mL was adopted to determine an abnormally increased serum level of SCC-Ag. The serum cancer antigen 125 (CA-125) level was also determined by radioimmunoassay. The cut-off value of 35 U/mL was adopted to determine abnormally increased serum level of CA-125. Patients were classified into two categories: negative (SCC-Ag <1.5 ng/mL and CA-125 <35 U/mL) and positive (SCC-Ag ≥1.5 ng/mL or CA-125 ≥35 U/mL).
The primary outcome measure was PI, which was confirmed by pathological examination. First, the chi-square test was performed to determine the relationship between parametrial invasion and each of the following pretreatment factors: (1) histological variant (SCC vs. non-SCC); (2) maximum tumor diameter (<25 mm vs. ≥25 mm); (3) pelvic lymph node enlargement (no vs. yes); (4) volume index (<5,000 mm3 vs. ≥5,000 mm3); and (5) tumor marker (negative vs. positive). Factors that achieved statistical significance in univariate analysis were subsequently included in multivariate analysis. Logistic regression analysis was used to select the independent risk factors for parametrial invasion. The statistical significance level was set at 0.05. Statistical analyses were performed with R version 3.2.0: A Language and Environment for Statistical Computing (R Core Team 2015).
Table 2 shows the clinicopathological characteristics of the 115 patients included in this study. Their median age was 41 years (range, 27 to 77 years). Sixty-five patients (57%) had SCC and 50 (43%) had non-SCC. The breakdown of non-SCC was: adenocarcinoma, 37; adenosquamous carcinoma, 9; and other types of carcinoma, 4. Eighteen patients (16%) were pathologically diagnosed with PI. Seventeen patients (15%) had lymph node metastasis (LNM). Seventy-one patients (62%) showed positive serum tumor markers before surgery. Sixty-seven patients (58%) received no adjuvant treatment and 45 patients (39%) received adjuvant platinum-based chemotherapy.
Table 3 shows the relationship between pretreatment variables and PI. The percentage of patients with PI significantly increased as the MRI-based tumor diameter increased (p<0.001); 1.5% (tumor diameter, <20 mm), 8.7% (tumor diameter, 20 to 25 mm), 27.3% (tumor diameter, 25 to 30 mm), 66.7% (tumor diameter, 30 to 35 mm), and 80.0% (tumor diameter, 35 to 40 mm). The percentage of patients with PI significantly increased as volume index increased (p<0.001); 1.4% (tumor volume, <5,000 mm3), 11.8% (tumor volume, 5,000 to 10,000 mm3), 50.0% (tumor volume, 10,000 to 20,000 mm3), 70.0% (tumor volume, 20,000 to 30,000 mm3), and 75.0% (tumor volume, ≥30,000 mm3). The rate of PI was significantly higher in patients with positive results for serum tumor marker than in those with negative results (31.8% vs. 5.6%, p<0.001).
Table 4 shows the result of logistic regression analysis of pretreatment factors relating to PI. Multivariate analysis confirmed that MRI-based tumor diameter (odds ratio [OR], 9.9; 95% confidence interval [CI], 2.1 to 48.1), volume index (OR, 13.3; 95% CI, 1.4 to 125.0), and serum tumor marker (OR, 5.7; 95% CI, 1.3 to 25.1) were independent risk factors for PI. Receiver operating characteristic curves were constructed to evaluate the performance of MRI-based tumor diameter, volume index, serum level of SCC-Ag and serum level of CA-125 for predicting PI (Fig. 1). Area under the curve (AUC) of tumor diameter, volume index, SCC-Ag, and CA-125 was 0.925 (95% CI, 0.869 to 0.981; p<0.001), 0.921 (95% CI, 0.860 to 0.982; p<0.001), 0.768 (95% CI, 0.644 to 0.892; p<0.001), and 0.528 (95% CI, 0.347 to 0.709; p=0.764), respectively. AUC of combination criteria consisting of tumor diameter, volume index, and serum tumor markers was 0.933 (95% CI, 0.893 to 0.974; p<0.001), which indicated the best performance. Table 5 shows actual frequencies of PI according to these risk factors. Of 53 patients with no risk factors for PI, none had PI.
While radical trachelectomy has achieved satisfactory oncological outcomes in selected patients with early-stage cervical cancer, it has had unsatisfactory obstetrical outcomes [4]. Because the number of patients with invasive cervical cancer is increasing among young women who long for pregnancy and childbirth, less-invasive surgery should be considered as an alternative therapy. Previous studies have confirmed that tumor diameter [1315161825], depth of cervical invasion [810112526], lymphovascular space invasion (LVSI) [781011151825], and pelvic LNM [8111325] are pathological risk factors for PI in cervical cancer.
Some low-risk criteria for PI have been proposed by optionally combining the above-mentioned risk factors. When tumor diameter <20 mm, depth of cervical invasion <10 mm, negative LVSI, or negative LNM were considered as a component of low-risk criteria, the rate of PI was reported to be <2.0% [1112141822]. However, surgery is necessary to identify the depth of cervical invasion or LVSI. In contrast, MRI seems to have become a reliable source of information regarding risk of PI. MRI-based tumor size can be a substitute for a histopathologically measured tumor. Kodama et al. [16] demonstrated a significant correlation between MRI-based and histopathological tumor size in stage IB1 cervical cancer (r=0.787, p<0.001). Although the percentage of PI is 0% to 4.5% in small (<20 mm) cervical cancer [1415161718], it is 0% to 1.1% in MRI-based, small (<20 mm) cervical cancer [91016]. MRI-based tumor diameter <20 mm can be a sufficient index for low-risk PI. Patients who meet these criteria might be candidates for less-invasive surgery.
We focused on patients with tumor diameter 20 to 25 mm, which accounts for 20% to 30% of all stage IB1 cervical cancer in our study. It is possible to extend the indication range for less-invasive surgery by assessing tumor volume, serum tumor marker, and tumor diameter in one direction. In our study, a low-risk of PI was confirmed as a tumor diameter <25 mm, a small volume index, and negativity for serum tumor markers (SCC-Ag and CA-125). No PI was seen in patients meeting these criteria, accounting for 47% of all stage IB1 cervical cancer in the present study.
Lee et al. [27] focused on patients with microscopic stage IB1 disease, namely, the cancer can be seen only with a microscope. They suggested a unique criterion for less radical surgery, as patients with no demonstrable lesions on postconization MRI in microscopic stage IB1 cervical cancer [27]. The rate of PI was 1.4% (1/74) for invisible tumors on MRI and 11.8% (6/51) for MRI-visible tumors. They claimed that less radical surgery, namely, omission of parametrectomy is feasible in accordance with their criteria. In the present study, 17 patients (14.5%) were diagnosed by conization, thus the above-mentioned criteria should be applicable to such patients.
Tumor volume or serum tumor markers may play an auxiliary role for tumor diameter to confirm a low-risk group for PI. Chang et al. [13] also proposed unique low-risk criteria for PI. They showed that the percentage of PI was 1.1% in patients with tumor diameter <30 mm and negativity for serum SCC-Ag [13]. Moreover, intraoperative assessment including sentinel lymph node biopsy can be promising. Klat et al. [23] showed that no PI was identified in patients with tumor diameter <20 mm and negative sentinel LNM.
Our study had some limitations. First, the number of patients was too small to power conclusive results. Our study inevitably had selection bias because of its retrospective nature and being a single-institution study. Second, reproducibility with regard to measurement of tumor diameter or tumor volume, namely interobserver variability has not yet been validated.
In conclusion, MRI-based tumor diameter ≥25 mm, volume index ≥5,000 mm3, and positive serum tumor markers SCC-Ag ≥1.5 ng/mL or CA-125 ≥35 U/mL were independent risk factors for PI in a Japanese cohort. Less radical surgery may become one of the treatment options for patients with stage IB1 cervical cancer who do not have any of these risk factors.
Figures and Tables
 | Fig. 1(A) Receiver operating characteristic (ROC) curves of tumor diameter, volume index, and serum tumor markers (SCC-Ag and CA-125) for predicting parametrial involvement (PI). (B) ROC curve of combination of tumor diameter, volume index, and serum tumor markers for predicting PI. |
Table 1
Distribution of initial treatment for 421 patients with cervical cancer at the Hokkaido Cancer Center between January 2008 and June 2014

Table 2
Clinical characteristics of 115 patients with cervical cancer treated with radical hysterectomy/trachelectomy with pelvic lymphadenectomy
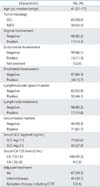
Table 3
A relationship between pretreatment variables and parametrial involvement

Table 4
Logistic regression analysis of pretreatment factors relating to parametrial involvement

Table 5
Rates of parametrial involvement by pretreatment risk factors for parametrial involvement

References
1. Randall ME, Michael H, Long H 3rd, Tedjarati S. Uterine cervix. In : Barakat RR, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2009. p. 623–681.
2. National Comprehensive Cancer Network. NCCN Clinical Guideline in Oncology. Cervical Cancer Version 2. 2015 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;c2015. cited 2015 Jul 8. Available from: http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
3. Green TH Jr, Meigs JV, Ulfelder H, Curtin RR. Urologic complications of radical Wertheim hysterectomy: incidence, etiology, management, and prevention. Obstet Gynecol. 1962; 20:293–312.
4. Plante M. Evolution in fertility-preserving options for early-stage cervical cancer: radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. Int J Gynecol Cancer. 2013; 23:982–989.
5. Benedetti-Panici P, Maneschi F, D'Andrea G, Cutillo G, Rabitti C, Congiu M, et al. Early cervical carcinoma: the natural history of lymph node involvement redefined on the basis of thorough parametrectomy and giant section study. Cancer. 2000; 88:2267–2274.
6. Puente R, Guzman S, Israel E, Poblete MT. Do the pelvic lymph nodes predict the parametrial status in cervical cancer stages IB-IIA? Int J Gynecol Cancer. 2004; 14:832–840.
7. Silva-Filho AL, Reis FM, Traiman P, Pedrosa MS, Miranda D, Triginelli SA. Clinicopathological features influencing pelvic lymph node metastasis and vaginal and parametrial involvement in patients with carcinoma of the cervix. Gynecol Obstet Invest. 2005; 59:92–96.
8. Steed H, Capstick V, Schepansky A, Honore L, Hiltz M, Faught W. Early cervical cancer and parametrial involvement: is it significant? Gynecol Oncol. 2006; 103:53–57.
9. Kamimori T, Sakamoto K, Fujiwara K, Umayahara K, Sugiyama Y, Utsugi K, et al. Parametrial involvement in FIGO stage IB1 cervical carcinoma diagnostic impact of tumor diameter in preoperative magnetic resonance imaging. Int J Gynecol Cancer. 2011; 21:349–354.
10. Lee JY, Youm J, Kim TH, Cho JY, Kim MA, Suh DH, et al. Preoperative MRI criteria for trials on less radical surgery in Stage IB1 cervical cancer. Gynecol Oncol. 2014; 134:47–51.
11. Covens A, Rosen B, Murphy J, Laframboise S, DePetrillo AD, Lickrish G, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. 2002; 84:145–149.
12. Wright JD, Grigsby PW, Brooks R, Powell MA, Gibb RK, Gao F, et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007; 110:1281–1286.
13. Chang SJ, Bristow RE, Ryu HS. A model for prediction of parametrial involvement and feasibility of less radical resection of parametrium in patients with FIGO stage IB1 cervical cancer. Gynecol Oncol. 2012; 126:82–86.
14. Frumovitz M, Sun CC, Schmeler KM, Deavers MT, Dos Reis R, Levenback CF, et al. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet Gynecol. 2009; 114:93–99.
15. Coutant C, Cordier AG, Guillo E, Ballester M, Rouzier R, Darai E. Clues pointing to simple hysterectomy to treat early-stage cervical cancer. Oncol Rep. 2009; 22:927–934.
16. Kodama J, Fukushima C, Kusumoto T, Nakamura K, Seki N, Hongo A, et al. Stage IB1 cervical cancer patients with an MRI-measured tumor size < or = 2 cm might be candidates for less-radical surgery. Eur J Gynaecol Oncol. 2013; 34:39–41.
17. Kinney WK, Hodge DO, Egorshin EV, Ballard DJ, Podratz KC. Identification of a low-risk subset of patients with stage IB invasive squamous cancer of the cervix possibly suited to less radical surgical treatment. Gynecol Oncol. 1995; 57:3–6.
18. Gemer O, Eitan R, Gdalevich M, Mamanov A, Piura B, Rabinovich A, et al. Can parametrectomy be avoided in early cervical cancer? An algorithm for the identification of patients at low risk for parametrial involvement. Eur J Surg Oncol. 2013; 39:76–80.
19. Biliatis I, Kucukmetin A, Patel A, Ratnavelu N, Cross P, Chattopadhyay S, et al. Small volume stage 1B1 cervical cancer: Is radical surgery still necessary? Gynecol Oncol. 2012; 126:73–77.
20. Bouchard-Fortier G, Reade CJ, Covens A. Non-radical surgery for small early-stage cervical cancer. Is it time? Gynecol Oncol. 2014; 132:624–627.
21. Rob L, Pluta M, Strnad P, Hrehorcak M, Chmel R, Skapa P, et al. A less radical treatment option to the fertility-sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol Oncol. 2008; 111:2 Suppl. S116–S120.
22. Stegeman M, Louwen M, van der Velden J, ten Kate FJ, den Bakker MA, Burger CW, et al. The incidence of parametrial tumor involvement in select patients with early cervix cancer is too low to justify parametrectomy. Gynecol Oncol. 2007; 105:475–480.
23. Klat J, Sevcik L, Simetka O, Graf P, Dvorackova J, Kraft O. What is the risk for parametrial involvement in women with early-stage cervical cancer with tumour <20 mm and with negative sentinel lymph nodes? Aust N Z J Obstet Gynaecol. 2012; 52:540–544.
24. Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994; 190:807–811.
25. Landoni F, Bocciolone L, Perego P, Maneo A, Bratina G, Mangioni C. Cancer of the cervix, FIGO stages IB and IIA: patterns of local growth and paracervical extension. Int J Gynecol Cancer. 1995; 5:329–334.
26. Inoue T. Prognostic significance of the depth of invasion relating to nodal metastases, parametrial extension, and cell types. A study of 628 cases with Stage IB, IIA, and IIB cervical carcinoma. Cancer. 1984; 54:3035–3042.
27. Lee JY, Youm J, Kim JW, Cho JY, Kim MA, Kim TH, et al. Identifying a low-risk group for parametrial involvement in microscopic stage IB1 cervical cancer using criteria from ongoing studies and a new MRI criterion. BMC Cancer. 2015; 15:167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download