Abstract
Objective
This study reports our initial experience of robotic high para-aortic lymph node dissection (PALND) with high port placement using same port for pelvic surgery in cervical and endometrial cancer patients.
Methods
Between July 2013 and January 2014, we performed robotic high PALND up to the left renal vein during staging surgeries. With high port placement and same port usage for pelvic surgery, high PALND was successfully performed without repositioning the robotic column. All data were registered consecutively and analyzed retrospectively.
Results
All patients successfully underwent robotic high PALND, followed by hysterectomy and pelvic lymph node dissection. Median age was 45 years (range, 39 to 51 years) and median body mass index was 22 kg/m2 (range, 19.3 to 23.1 kg/m2). Median operative time for right PALND and left PALND was 37 minutes (range, 22 to 65 minutes) and 44 minutes (range, 36 to 50 minutes), respectively. Median number of right and left para-aortic lymph node by pathologic report was 12 (range, 8 to 15) and 13 (range, 5 to 26).
Lymph node status is an important independent prognostic factor in cancer of the genital tract [1]. In gynecologic cancer, surgical excision of para-aortic lymph node (PALN) for pathologic review is important to decide the treatment plan. In addition, removal of metastatic lymph nodes may have some therapeutic effect [2]. Para-aortic lymph node dissection (PALND) is selectively indicated in some patients with gynecologic cancer. The extent of PALND can be divided into infrarenal ("high") and inframesenteric nodes. Determining the extent of PALND has been a controversial issue in gynecologic cancer.
It has been believed that although robotic surgery facilitate minimally invasive oncological procedures, the current robotic system prohibits operating both in the pelvis and upper abdomen because of the limitations in arm mobility [3]. Several studies have reported that for removal of the infrarenal aortic nodes adjacent to the left renal vessels, the robotic column must be repositioned at the patient's head for a transperitoneal midline approach [3], at the patient's right for an extraperitoneal approach [4], and at the patient's left for a transperitoneal left lateral approach [5], with additional trocar placement for each one of these approaches. In addition, "hybrid" procedure which performes conventional laparoscopy as an adjunct can be another option. With the methods mentioned above, total operation time may be elongated and could be a technical challenge to the surgeon and anesthesia team.
However, operating both in pelvis and high para-aortic region with the same port placement is not impossible, although it is not technically easy. All robotic ports and one assistant port were located above the level of umbilicus to access both high para-aortic lymph node and pelvis.
Here, we report the cases of five patients with cervical and endometrial cancer who successfully underwent robotic high PALND using same port placement for pelvic surgery.
We retrospectively analyzed five patients who had been diagnosed of early cervical cancer or endometrial cancer at Samsung Medical Center from July 2013 to January 2014. Diagnostic tests such as pelvic examination, pelvic magnetic resonance imaging and abdominal computed tomography (CT), cystoscopy (except for endometrial cancer), chest X-ray, electrocardiogram, blood test were basically performed in all patients. We performed hysterectomy (type III hysterectomy in cervical cancer) and bilateral pelvic lymph node dissection using da Vinci robotic system as conventional laparoscopic method. Surgery was performed by one surgeon (TJK).
All patients with endometrial cancer had risk factors indicating PALND including myometrial invasion >50%, non-endometroid pathology, large tumor size, and tumor grade 3. A patient with cervical cancer had positive PALN on CT scan. All data were registered consecutively using Microsoft Excel program and included patient age, body mass index (BMI), tumor grade, operation time, lymph node count and status and intraoperative complication. Operation time was recorded in operation records section by section.
Patients were positioned on the operating table as described by Holloway and Ahmad [6] with few modifications. 30° da Vinci angled scope was inserted through 12 mm middle trocar placed approximately 23 to 25 cm cephalad to the symphysis pubis. Ancillary trocars were placed in the supine position as follows: (1) 8 mm robotic port (first arm) was placed on the patient's right side, 8 to 10 cm lateral and 1 to 2 cm inferior to the camera port; (2) 8 mm robotic port (second arm) was placed on the patient's left side, 8 to 10 cm lateral and 1 to 2 cm inferior to the camera port; (3) 8 mm robotic port (third arm) was placed 8 to 10 cm lateral, 1 to 2 cm inferior to the second arm; and (4) assistant trocar for traction of the small bowel (duodenum) was placed 1 cm inferior to the subcostal margin on the right mid-clavicular line. All trocars were placed above or near umbilicus level (Fig. 1). In order to avoid arm collision or fighting, we tried to maintain at least a 7 to 8 cm distance between the ports.
Steep Trendelenburg positioned to 25° was done with washable gel-pads placed under the sacrum, shoulders. A tension tape positioned over towels on the patients' clavicles and shoulders. RUMI manipulator and Koh colpotomiser system (Cooper Surgical, Shelton, CT, USA) was set for uterine mobilization. Central docking of the robotic column and two surgical gauze insertion was done before the beginning of the surgery. Bipolar grasper (Fenestrated Bipolar Forceps, Endowrist Instrument; Intuitive Surgical, Sunnyvale, CA, USA) and monopolar scissors (Endowrist Instrument; Intuitive Surgical) were introduced through left and right robotic trocars respectively. In the third arm, Prograsp Forceps (Intuitive Surgical) was used. We performed PALND before initiating the pelvic surgery. We began from the right side and conducted from caudal to cephalad direction. In case of the right PALND, we used fenestrated bipolar forceps in the first arm and monopolar scissor in the second arm. We dissected the lymph node around inferior vena cava (IVC) from patient's left to right side. In case of right PALND, it is crucial for the assistant to tract duodenum to the direction of patient's head in order to optimize the surgical view and tract right ureter lateral side by Prograsp near the common iliac artery area. During the left PALND, we conducted from caudal to cephalad direction as same as the right side. In this case, it is critical to use Prograsp and tract the peritoneum near inferior mesenteric artery (IMA). Traction of small bowel through assistant arm in the case of left high PALND is also important as the right side. Intraoperative and postoperative photo of our port placement and intraoperative image scan was provided at Figs. 1,2,3. Postoperative care followed the standard protocol for patients who undergo conventional laparoscopy.
The statistical values in this article were presented as medians (Table 1). All surgeries were completed by the robotics without conversion. All patients tolerated the surgery and none required readmission or reoperation in the 1-month postoperative period. All patients successfully underwent robotic high PALND using same port placement for pelvic surgery. All patients underwent concomitant robotic hysterectomy and pelvic lymphadenectomy (data not shown). Median age was 45 years (range, 39 to 51 years) and median BMI was 22 kg/m2 (range, 19.3 to 23.1 kg/m2). Median operative time for right PALND and left PALND 37 was minutes (range, 22 to 65 minutes) and 44 minutes (range, 36 to 50 minutes) respectively. Median number of right and left PALN by postoperative pathologic report was 12 (median, 8 to 15) and 13 (median, 5 to 26). The harvested number of pelvic lymph nodes of patient 1 to patient 5 was 7, 18, 12, 12, and 15, respectively. No blood transfusions occurred. There was one intraoperative complication of IVC tear with primary repaired with Prolene 5-0. Repair time was included in the data.
We report five initial cases which successfully completed robotic high PALND with same port placement in the pelvic surgery. The peculiarity is that it does not require additional trocar placement and readjustment of the robotic column. By using five trocar with high port placement, we performed pelvic surgery and high PALND simultaneously. Traction of the duodenum is important and technically challenging since angle of approach from high port placement is not so good as traditional laparoscopic approach with low port placement.
Although being controversial, it has been recommended to perform PALND to the level of the left renal vein to high risk endometrial caner paitents. Apart from the lymphatic metastasis routes between the uterus and the external iliac, obturator basins, a direct pathway may exist from the uterus to the aortic-node basins. It is considered to occur through lymphatics adjacent to the gonadal vessels within the infundibulopelvic ligament [7]. In node positive endometrial cancer patients, the aortic nodes were found to be involved in 67% of patients, and among those the left infrarenal group was involved in 77%. More importantly, among those with positive left infrarenal nodes, 60% had negative ipsilateral inframesenteric nodes and 71% had negative ipsilateral common iliac nodes [8]. Since the number of left infrarenal nodes is about twice the number of inframesenteric nodes and that lymphatic channels along the ovarian vessels bypass the inframesenteric nodes, the left infrarenal group can be involved despite negative inframesenteric nodes. Therefore, bilateral dissection of the PALN between the IMA and renal vein is essential for PALND.
Magrina et al. [3] reported that it is possible to remove the pelvic and inframesenteric aortic nodes even in the cases of some nodes above the IMA in patients with a normal BMI, with the robotic set up for pelvic surgery. However it is not possible to excise the nodal tissue from the left renal vein or immediately below it due to limitations of arm mobility [3]. But with same port usage for pelvic surgery, we successfully performed high PALND by robotics without repositioning the robotic column.
In order to conduct the surgery, which we used in this study, it is essential to clear the surgical view. There are two characteristics in our robotic set up. First, since the patient is taking steep Trendelenburg position and we conduct the lymph node dissection from caudal to cephalad, it causes lymphatic fluid to get collected on the surgical site, which can entail problems in the operation field of the high PALND. Inserting the gauze inside the abdominal cavity helps overcome this problem. Second, it is important for assistant to tract small bowel to clear the surgical view and secure the mobility of the surgical instruments. It is possible to use tagging traction suture and put away the small bowel from operation site by fixing the peritoneum on abdominal wall. In this case, the mobility of the surgical instruments through assistant port and robot ports become limited.
Obesity is associated with a 10-fold increased risk for endometrial cancer and is considered as an important factor to select laparoscopic or robotic procedures. Obese patients also have an increased risk of less complete lymph node dissection and laparotomy conversion [9]. Maintenance of exposure during aortic lymph node dissection and adequate ventilation with requirements for steep Trendelenburg positioning can be challenging for both surgeon and anesthesiologist [6]. BMI of the patients of this study were below 25 kg/m2. Korean patients with endometrial cancer were reported to have lower BMI (<25 kg/m2) than previous reports [10]. In this sense, it would worth to try robotic PALND in case of non-obese endometrial cancer.
Along the development of robotic surgery, the complex and fine dissection around great vessel has been enabled due to the three-dimensional visualization and articulating wristed instruments [11]. However, current robotic system has a limitation that it cannot access the whole abdomino-pelvic cavity. Several methods such as patient rotation or relocation of the robotic column have been introduced but these methods are time-consuming and technologically complicated which requires highly skilled surgeon and anesthesia team.
The methods which were introduced in this study are initial experience and have a few limitations. However, if we evolve this technique and conduct larger study for assessing the generalized feasibility and usefulness, it can be an excellent surgical option to supplement the existing methods in conducting robotic high PALND.
In conclusion, with high port placement, robotic high PALND with the same port placement used in pelvic surgery is feasible to non-obese patients. This method may be an attractive surgical and technical option for robotic PALND.
Figures and Tables
Fig. 1
The port placement (intraoperative and postoperative view). (A) The cephalad part is above the assistant port and the caudal part is below the umbilicus. (B) Left side is the cephalad part and right side is the caudal part.
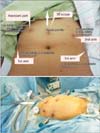
Fig. 2
Intraoperative view after completing left side para-aortic lymph node dissection to inferior mesenteric artery level. Inferior mesenteric artery originating from aorta is seen just above the suction.

Fig. 3
Intraoperative view after completing left side para-aortic lymph node dissection to left renal vein level. Left renal vein is seen at left side to the suction.
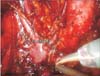
Table 1
Clinical data of five patients

References
1. Possover M, Krause N, Plaul K, Kuhne-Heid R, Schneider A. Laparoscopic para-aortic and pelvic lymphadenectomy: experience with 150 patients and review of the literature. Gynecol Oncol. 1998; 71:19–28.
2. Kjorstad KE, Kolbenstvedt A, Strickert T. The value of complete lymphadenectomy in radical treatment of cancer of the cervix, stage IB. Cancer. 1984; 54:2215–2219.
3. Magrina JF, Long JB, Kho RM, Giles DL, Montero RP, Magtibay PM. Robotic transperitoneal infrarenal aortic lymphadenectomy: technique and results. Int J Gynecol Cancer. 2010; 20:184–187.
4. Magrina JF, Kho R, Montero RP, Magtibay PM, Pawlina W. Robotic extraperitoneal aortic lymphadenectomy: development of a technique. Gynecol Oncol. 2009; 113:32–35.
5. Jacob KA, Zanagnolo V, Magrina JF, Magtibay PM. Robotic transperitoneal infrarenal aortic lymphadenectomy for gynecologic malignancy: a left lateral approach. J Laparoendosc Adv Surg Tech A. 2011; 21:733–736.
6. Holloway RW, Ahmad S. Robotic-assisted surgery in the management of endometrial cancer. J Obstet Gynaecol Res. 2012; 38:1–8.
7. Mariani A, Webb MJ, Keeney GL, Podratz KC. Routes of lymphatic spread: a study of 112 consecutive patients with endometrial cancer. Gynecol Oncol. 2001; 81:100–104.
8. Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008; 109:11–18.
9. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009; 27:5331–5336.
10. Lee EJ, Kim TJ, Choi CH, Lee JW, Lee JH, Bae DS, et al. Uterine endometrial carcinoma: 10 years' experience with long-term follow-up at a single Korean institution. Gynecol Obstet Invest. 2012; 74:313–319.
11. Fastrez M, Vandromme J, George P, Rozenberg S, Degueldre M. Robot assisted laparoscopic transperitoneal para-aortic lymphadenectomy in the management of advanced cervical carcinoma. Eur J Obstet Gynecol Reprod Biol. 2009; 147:226–229.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download