Abstract
Objective
To investigate the rate, predictors of lymph node metastasis (LNM) and pattern of recurrence in clinically early stage endometrial cancer (EC) with positive lymphovascular space invasion (LVSI).
Methods
Women with clinically early stage EC and positive LVSI 2005 to 2012 were identified. Kaplan-Meier curves and logistic regression models were used.
Results
One hundred forty-eight women were identified. Of them, 25.7% had LNM (21.7% pelvic LNM, 18.5% para-aortic LNM). Among patients with LNM who had both pelvic and para-aortic lymphadenectomy, isolated pelvic, para-aortic and both LNM were noted in 51.4%, 17.1%, and 31.4% respectively. Age and depth of myometrial invasion were significant predictors of LNM in LVSI positive EC. Node positive patients had high recurrence rate (47% vs. 11.8%, p<0.05) especially distant (60.9% vs. 7.9%, p<0.001) and para-aortic (13.2% vs. 1.8%, p=0.017) recurrences compared to node negative EC. LNM was associated with lower progression-free survival (p=0.002) but not overall survival (p=0.73).
In clinically early stage endometrial cancer (EC), lymphovascular space invasion (LVSI) has repeatedly been associated with disease spread and poor outcomes. The presence of LVSI is associated with lymph node metastasis (LNM), a critical factor with regards to staging, prognosis and treatment planning of clinically early stage EC [123456]. Further, the presence of LVSI is associated with lymphatic and hematogenous spread to distant sites [789]. Even in women with surgically staged EC confined to the uterus (negative lymph nodes), the positive association between LVSI and recurrence risk has been reported [13101112]. While the status of pelvic and para-aortic lymph nodes is critical to staging and prognosis, lymphadenectomy is often omitted from surgical staging procedures if the surgeon feels that the risk of LNM is low based on preoperative and intraoperative findings. Unfortunately LVSI is often not revealed until the time of final pathology. In the presence of LVSI, authors report rates of pelvic lymph node metastases ranging from 25% to 45% [1345]. Some authors argue that LVSI on a uterine specimen is sufficient to warrant returning to the operating room for completion lymphadenectomy or to recommend adjuvant radiation and/or systemic therapies [13413]. While it is understood that LVSI conveys an increased risk of LNM, few series have detailed the risk and predictors of LNM in women with clinically early stage EC with positive LVSI.
While the presence of LNM carries with it significant implications for treatment planning and survival, the pattern of recurrence in LVSI positive patients stratified by lymph node status has not been thoroughly detailed to date. Characterizing and understanding the behavior of these tumors may have profound implications when discussing treatment options and prognosis. The objective of this study is to investigate the rate and the predictors of LNM in clinically early stage EC with positive LVSI. Further, the rate and pattern of recurrence of node negative and node positive EC with positive LVSI were measured.
Patients with endometrial adenocarcinomas who underwent surgical treatment at the Cleveland Clinic Health System for the period from 2005 to 2012 were identified. Approval to conduct this study was obtained from Cleveland Clinic Institutional Review Board for Human Subjects Protection. Patients with clinical stage I endometrioid or mixed (at least 50% endometrioid) endometrial adenocarcinoma who had undergone total hysterectomy and bilateral salpingo-oophorectomy and lymphadenectomy with pelvic and/or para-aortic lymphadenectomy and have positive LVSI were included in the study. Patients with negative LVSI, other histology types, preoperative evidence of extra-uterine disease, stage IV cancer or those who did not undergo surgery were excluded. Demographic, clinicopathologic, surgical/adjuvant treatment, and follow-up data were extracted from pathology reports and medical records of all patients. Following surgery, patients underwent radiation therapy, chemotherapy or combined chemotherapy, and radiation therapy. Adjuvant radiation therapy was delivered by external beam radiotherapy and/or brachytherapy.
The primary endpoints were the rate and predictors of LNM in patients with clinically early EC with positive LVSI. Secondary endpoints were rate and pattern of recurrence in node positive compared to node negative EC with positive LVSI.
Associations between categorical and continuous variables were assessed using chi-square test, Fisher exact, and Welch t-test. Logistic regression models were used to quantify the associations of independent predictors with LNM. Factors entered in the multivariable model included age at diagnosis, grade and depth of myometrial invasion (grade was then removed from the final model as it was not significantly associated with LNM). The Kaplan-Meier methods were used to estimate progression-free survival and overall survival. Overall survival was calculated from date of diagnosis to date of death or last follow-up. Patients who are alive at the time of analysis were censored. Progression-free survival was calculated from date of diagnosis to date of progression/recurrence or last follow-up. R-Studio, integrated development environment for R version 0.97.551 (R-Studio, Boston, MA, USA) with R 3.1.0 was used for the statistical analyses.
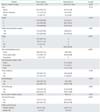
One hundred forty-eight patients were included in this study. Of them, 38 were found to have positive nodes (25.7%). Positive pelvic nodes and para-aortic nodes were found in 21.7% (32/147) and 18.5% (17/92). Among patients with positive nodes who had both pelvic and para-aortic lymphadenectomy, nodal metastasis was found in only pelvic, para-aortic and both pelvic/para-aortic in 51.4% (18/35), 17.1% (6/35), and 31.4% (11/35). Among the node positive group, 79% patients (30/38) received pelvic radiation and 74% (28/38) received systemic adjuvant therapy including 60.5% received chemotherapy (23/38) (Table 1). Demographic, clinical and treatment characteristics of patients with negative and positive nodes are shown in Table 1.
In multivariable analysis that included the entire cohort and adjusting for age, grade, and depth of myometrial invasion, predictors of any positive nodes were age (odds ratio [OR], 0.39; 95% confidence interval [CI], 0.2 to 0.71; p=0.003) and deep myometrial invasion (OR, 5.6; 95% CI, 2.6 to 13.1; p<0.001). For pelvic nodes, predictors of positive nodes were age (OR, 0.48; 95% CI, 0.25 to 0.88; p=0.022) and deep invasion (OR, 4.5; 95% CI, 2.1 to 10.7; p<0.001). For para-aortic nodes, predictors were age (OR, 0.3; 95% CI, 0.11 to 0.70; p=0.009) and deep invasion (OR, 8.2; 95% CI, 2.7 to 30.9; p<0.001).
The rate of positive nodes among grade 1, 2, and 3 patients was 11% (3/27), 36% (17/47), and 32.5% (13/40), respectively (p=0.05). Among patients with no or <50% invasion, the rate of positive nodes was 12% (6/49) compared to 40% (26/65; p=0.001) for patients with deep invasion (50% or more). Rates of positive nodes by grade and depth of myometrial invasion are listed in Table 2.
The rate of recurrence among node positive and node negative patients was 47% (18/38) versus 11.8% (13/110; p<0.001) respectively. The rates of vaginal, pelvic, and extrapelvic recurrences were 10.5% (4/38) versus 2.7% (3/110; p=0.05), 10.5% (4/38) versus 3.6% (4/110; p=0.10), and 26.3% (10/38) versus 6.4% (7/110; p=0.001), respectively. Among extrapelvic recurrences, the rates of distant, peritoneal, and para-aortic recurrences were 7.9% (3/38) versus 0.9% (1/110; p<0.001), 5.3% (2/38) versus 2.7% (3/110; p=0.82), and 13.2% (5/38) versus 1.8% (2/110; p=0.017), respectively.
Endometrial cancer is the fifth most common cancer in women worldwide, but the treatment of clinically early stage tumors remains elusive [1013]. As we continue to understand the natural history of this disease relative to various risk factors, it is often helpful to be able to counsel women regarding the risks and benefits of any additional surgeries or adjuvant therapies. Pooling a large historic cohort of women, we found that 25.7% of women with clinically early stage, LVSI positive EC had stage IIIC disease with positive para-aortic nodes found in almost 50% of those with positive nodes. Not surprisingly, LNM were associated a higher risk of recurrence; in particular, women with LNM experienced higher para-aortic and distant failures compared to women without LNM.
Cohn et al. [4] reported LNM in 38% of patients with LVSI positive EC. However, the study by Cohn et al. [4] included all histologic types and was not limited to endometrioid type. Further, the rate of para-aortic nodal metastasis was not specified [4]. The rate of LNM was lower in this study (25%), which is likely related to including only patients with pure or predominant endometrioid histology. Further, we were able to details the location of lymph nodes among those with positive nodes. Interestingly, positive para-arotic nodes was found in 48% of those with positive lymph nodes, which further confirm the importance of comprehensive surgical staging in these patient population. The data in this study further confirm the findings by Cohn et al. [4] about the impact of LVSI on risk of LNM beside other well known variables such as tumor grade and depth of myometrial invasion. The findings in this study are helpful in patient counseling and postoperative treatment decision in patients with positive LVSI who did not undergo surgical staging.
The overall recurrence rate in this study was 21% with higher rate as expected for those with positive lymph nodes compared to those with negative lymph nodes. Other authors have demonstrated the association between LVSI and pattern of recurrence. In a large series by Watari et al. [7], 10.5% of women with moderate or prominent LVSI experienced distant failure. Inoue et al. [8], reported that LVSI was associated with 21.3% chance of tumor recurrence, often at distant sites.
There is a paucity of data with regards to location of recurrence in stage IIIC women with LVSI. Interestingly in this study, LVSI positive EC with LNM, had a higher rate of para-aortic and distant failures. In recent years, the administration of chemotherapy has been favored, subsequent to results of Gynecologic Oncology Group (GOG) 122 and the survival benefit associated with chemotherapy compared to whole abdominal radiation therapy [14]. Based on our results, systemic therapy to prevent distant recurrences should be considered in women with stage IIIC, LVSI positive EC.
The conclusions of this study are limited by its retrospective nature and sample size. Because of small sample size of patients with positive lymph nodes, we lacked the power to investigate the role of postoperative adjuvant therapy on risk and pattern of recurrence. Further, given its retrospective nature, we lacked information on rationale for performing only pelvic lymphadenectomy on some patients. However, this study is one of the few studies detailing the risk of harboring positive lymph nodes and pattern of recurrence in this unique patient population.
While most women with EC are early stage and have excellent long term survival, clearly there are subgroups of women identifiable by pathologic and personal risk factors who are at a higher risk for LNM and disease relapse. Ultimately, patients with positive LVSI harbor high risk of LNM with subsequent risk of recurrence especially distant recurrence. Interestingly, LVSI positive patients with grade 2 to 3 disease have a high incidence of LNM even in those with ≤50% myometrial invasion. As we know, information on LVSI status can be obtained only by final pathology. Therefore, these data need to verified in future prospective study to decide whether routine lymphadenectomy can be omitted in only small faction of those with grade 1 with ≤50% myometrial invasion to account for the risk of positive LVSI. The other challenging question is what do with patients who did not undergo surgical staging but were found to have positive LVSI on final pathology. Based on our data, surgical staging or adjuvant treatment should be considered. Further, the high risk of distant recurrence in patients with stage IIIC LVSI positive EC, necessitate further investigation to identify the most effective treatment strategy to provide both local and distant control in this unique patient population.
Figures and Tables
Fig. 1
(A) Kaplan-Meier curves for progression-free survival in stage I to III endometrial cancer patients with positive lymphovascular space invasion stratified by node status. (B) Kaplan-Meier curves for overall survival in stage I to III endometrial cancer patients with positive lymphovascular space invasion stratified by node status.

Table 1
Demographic, clinical and treatment characteristics of clinically early stage endometrial cancer patients with positive lymphovascular space invasion stratified by lymph node status (n=148)
References
1. Hahn HS, Lee IH, Kim TJ, Lee KH, Shim JU, Kim JW, et al. Lymphovascular space invasion is highly associated with lymph node metastasis and recurrence in endometrial cancer. Aust N Z J Obstet Gynaecol. 2013; 53:293–297.
2. Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC. Predictors of lymphatic failure in endometrial cancer. Gynecol Oncol. 2002; 84:437–442.
3. Briet JM, Hollema H, Reesink N, Aalders JG, Mourits MJ, ten Hoor KA, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005; 96:799–804.
4. Cohn DE, Horowitz NS, Mutch DG, Kim SM, Manolitsas T, Fowler JM. Should the presence of lymphvascular space involvement be used to assign patients to adjuvant therapy following hysterectomy for unstaged endometrial cancer? Gynecol Oncol. 2002; 87:243–246.
5. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987; 60:8 Suppl. 2035–2041.
6. Mariani A, Webb MJ, Keeney GL, Haddock MG, Aletti G, Podratz KC. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol. 2002; 87:112–117.
7. Watari H, Mitamura T, Moriwaki M, Hosaka M, Ohba Y, Sudo S, et al. Survival and failure pattern of patients with endometrial cancer after extensive surgery including systematic pelvic and para-aortic lymphadenectomy followed by adjuvant chemotherapy. Int J Gynecol Cancer. 2009; 19:1585–1590.
8. Inoue Y, Obata K, Abe K, Ohmura G, Doh K, Yoshioka T, et al. The prognostic significance of vascular invasion by endometrial carcinoma. Cancer. 1996; 78:1447–1451.
9. Mariani A, Webb MJ, Keeney GL, Calori G, Podratz KC. Hematogenous dissemination in corpus cancer. Gynecol Oncol. 2001; 80:233–238.
10. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004; 92:744–751.
11. Mariani A, Webb MJ, Keeney GL, Lesnick TG, Podratz KC. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002; 87:274–280.
12. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991; 40:55–65.
13. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer;2013. cited 2015 Jan 19. Available from: http://globocan.iarc.fr.
14. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:36–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download