Abstract
Objective
To compare the diagnostic performance of six different approaches for assessing myometrial infiltration using ultrasound in women with carcinoma of the corpus uteri.
Methods
Myometrial infiltration was assessed by two-dimensional (2D) transvaginal or transrectal ultrasound in 169 consecutive women with well (G1) or moderately (G2) differentiated endometrioid type endometrial carcinoma. In 74 of these women three-dimensional (3D) ultrasound was also performed. Six different techniques for myometrial infiltration assessment were evaluated. The impression of examiner and Karlsson's criteria were assessed prospectively. Endometrial thickness, tumor/uterine 3D volume ratio, tumor distance to myometrial serosa (TDS), and van Holsbeke's subjective model were assessed retrospectively. All subjects underwent surgical staging within 1 week after ultrasound evaluation. Definitive histopathological data regarding myometrial infiltration was used as gold standard. Sensitivity and specificity for all approaches were calculated and compared using McNemar test.
Results
The impression of examiner and subjective model performed similarly (sensitivity 79.5% and 80.5%, respectively; specificity 89.6% and 90.3%, respectively). Both methods had significantly better sensitivity than Karlsson's criteria (sensitivity 31.8%, p<0.05) and endometrial thickness (sensitivity 47.7%, p<0.05), and better specificity than tumor/uterine volume ratio (specificity 28.3%, p<0.05) and TDS (specificity 41.5%, p<0.05).
Conclusion
Subjective impression seems to be the best approach for assessing myometrial infiltration in G1 or G2 endometrioid type endometrial cancer by transvaginal or transrectal ultrasound. The use of mathematical models and other objective 2D and 3D measurement techniques do not improve diagnostic performance.
Myometrial infiltration is one of the most important prognostic factors in endometrial cancer [1]. The risk of lymph node metastases increases significantly, when endometrial cancer infiltrates deeper than half the myometrium [2]. On the contrary, when the tumor infiltrates less than half myometrium the benefit of routine lymphadenectomy in well-differentiated (G1) or moderately-differentiated (G2) endometrioid type cancer is unclear [3].
Current practice in most institutions includes intraoperative assessment of the myometrial infiltration after uterus removal to determine whether lymphadenectomy may be avoided [45]. However, intraoperative assessment is not available in all centers and may increase the cost and length of surgery. For this reason, preoperative assessment of myometrial infiltration by imaging techniques would be advisable.
Transvaginal ultrasound has been used for many years for assessing myometrial infiltration in endometrial cancer. Since the pioneer paper from Cacciatore et al. [6] many studies have been reported using examiner's impression [7891011] or more objective measurements [121314]. More recently, mathematical models [1516] and three-dimensional (3D) ultrasound haven been also proposed [1718].
The aim of this study is to compare the diagnostic performance of six different approaches for assessing myometrial infiltration using transvaginal/transrectal ultrasound in women with G1 or G2 endometrioid type carcinoma of the corpus uteri.
The study comprises a series of 169 consecutive women diagnosed as having a G1 or G2 endometrioid type endometrial carcinoma by preoperative biopsy managed at our institution between January 1995 and October 2014. Institutional Review Board approval was obtained and all patients gave oral informed consent. This series also constitutes the basis of another report [19].
Scanning protocol was the same throughout the entire study period. After inserting the endovaginal probe into the vagina or the rectum, the uterus was firstly scanned thoroughly in the longitudinal plane from side to side. Then, vaginal probe was tilted 90° and the uterus was scanned in the transverse plane from cervix to fundus. Then the uterus was measured in the three orthogonal planes, as well as the endometrial thickness at the level of its maximum thickness in the longitudinal plane. Color Doppler mapping was also performed, but this information was not taken into consideration for assessing myometrial infiltration. In case of transrectal ultrasound previous cleansing of the rectum was done by simple rectal enema.
By January 2003, 3D ultrasound became available at our institution. Since then, all women included in the study also underwent 3D ultrasound according to a defined scanning protocol elsewhere described [17]. At least one 3D volume of the uterus was stored in all cases and subsequently analyzed in a personal computer using 4D view software (GE Medical Systems, Little Chalfont, UK).
Several ultrasound machines available were used through study period, all of them equipped with endovaginal probes with frequencies ranging from 5 to 10 MHz. The sonographer was aware of endometrial biopsy result.
Myometrial infiltration depth was subjectively estimated by looking at the point in which myometrial-endometrial interface was not clearly identified and then by looking at the supposedly tumor-free myometrial wall at this point. By using the opposite myometrial wall as a comparison, if a marked asymmetry was found, deep (≥50%) infiltration was stated; if myometrial thickness was similar in both myometrial walls, superficial (<50%) infiltration was stated. All ultrasound examinations were performed or supervised by one examiner (JLA). This data was collected prospectively and was available for all 169 women.
Objective measurement criteria for assessing myometrial infiltration, Karlsson's criteria, were defined as the ratio between maximal anteroposterior diameter of the endometrial lesion and the uterine anterior-posterior diameter, measured both in the sagittal plane [8]. A ratio ≥50% indicates a myometrial infiltration ≥50% (Fig. 1) and a ratio <50% indicates a myometrial infiltration <50% (Fig. 2). This data was collected prospectively and was available for 166 women. Endometrium was not measurable in three cases.
This data was collected prospectively and was available for 166 women. Diagnostic performance was analyzed retrospectively. We used a cut-off of ≥18 mm for predicting ≥50% myometrial infiltration as suggested by Mascilini et al. [18].
We retrospectively calculated tumor/uterine volume ratio from stored 3D volumes using the same methodology described by Mascilini et al. [18] (Fig. 3). This data was available in 74 women who underwent 3D ultrasound. We used a cut-off of ≤0.099 for predicting >50% myometrial infiltration as suggested by Mascilini et al. [18].
We retrospectively assessed with this model using the formula as proposed: z=-2.6276+1.1458×(preoperative grading)+2.2514×(subjective impression). We used an estimated probability of ≥0.50 for predicting ≥50% myometrial infiltration as suggested [16]. This data was available in 155 women. The model could not be applied in 12 cases because the tumor grade was not defined in preoperative biopsy.
One of the authors (RO) who was unaware of clinical data of the subjects, two-dimensional (2D) ultrasound assessments and pathological results performed all 3D assessments.
All subjects underwent surgical staging within 1 week after ultrasound evaluation. Definitive histopathological data regarding myometrial infiltration was used as gold standard. The pathologists were unaware of ultrasound examination. Tumor stage was stated according to the International Federation of Gynecology and Obstetrics classification [20].
Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy were calculated for all approaches. Sensitivity and specificity were compared using McNemar test. A p<0.05 was considered as statistically significant. SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used. We did not assess intra- and interobserver reproducibility of any of these methods.
Mean age was 60.7 years (standard deviation 10.3), ranging from 32 to 91 years. One hundred and thirty-eight women (81.7%) were postmenopausal and 31 women (18.3%) were premenopausal.
Preoperative tumor grade was G1 in 120 cases (71.0%) and G2 in 37 cases (21.9%). In 12 cases (7.1%) tumor grade was not defined in preoperative biopsy, but we decided not to exclude them from the study.
Definitive histologic diagnosis revealed myometrial infiltration of ≥50% in 44 cases (26.0%) and <50% in 125 cases (74.0%). In three cases no tumor was found at definitive histologic analysis; these cases were considered as having no infiltration and were not excluded. In three cases definitive histologic type was carcinosarcoma. We did not exclude these cases from this study. Definitive histologic grades were as follows: G1 (n=100), G2 (n=51), and G3 (n=15). Tumor stages were as follows: stage IA (n=116), stage IB (n=23), stage II (n=1), stage IIIA (n=9), stage IIIB (n=2), stage IIIC1 (n=9), stage IIIC2 (n=3), and stage IV (n=3).
Table 1 shows the myometrial infiltration according to the impression of examiner, Karlsson's criteria, endometrial thickness criteria, tumor/uterine 3D volume ratio, TDS, and subjective model, respectively.
Diagnostic performances for all six methods are shown in Table 2. We observed that the impression of examiner and subjective model perform similarly with no difference in terms of sensitivity and specificity. Both methods had significantly better sensitivity than Karlsson's criteria and endometrial thickness, and better specificity than tumor/uterine volume ratio and TDS.
In order to assess all six methods in the same set of patients we performed a subanalysis in the 74 patients in whom all approaches could be used. The results were statistically similar except for endometrial thickness (Table 3).
In this study we have compared up to six different approaches for assessing myometrial infiltration using transvaginal or transrectal ultrasound. Our original aim was to compare the impression of examiner with the objective approach proposed by Karlsson [8]. However, after learning the papers from Mascilini et al. [18] and van Holsbeke et al. [16], we decided to compare four more approaches. In this context, it is plausible that we have prospectively compared two different approaches, namely the impression and Karlsson's criteria, and that we have performed a retrospective external validation with all other approaches.
Our results are in agreement with two other studies [1618] so far reported comparing different approaches for assessing myometrial infiltration in endometrial cancer by transvaginal ultrasound: the impression performs as good or even better than objective measurement techniques. We found that sensitivity and specificity for the impression was 80% and 90%, respectively. Other authors have reported similar figures, with sensitivity ranging from 71% to 84% and specificity ranging from 72% to 89% [910111618].
The impression of examiner is inherently subjective and; therefore, reproducibility is a crucial issue. A significant limitation of our study is that we did not assess intra- and interobserver reproducibility and this might affect the generalization of these results. However, a recent paper form Eriksson et al. [21] has shown that the reproducibility of the impression for assessing myometrial infiltration in 53 cases of endometrial cancer using offline video-clips analyzed by 18 different examiners was good in most of pair comparisons.
Regarding objective measurement techniques, we found that Karlsson's criteria performed significantly poorer than subjective impression in terms of sensitivity (32% vs. 80%). This was also observed by van Holsbeke et al. (47% vs. 73%) [16]. However, previous studies from different authors reported better results for Karlsson's criteria, with sensitivity ranging from 89% to 93% [22232425]. These discordant results could be explained with difference in patient selection. In van Holsbeke's study and ours, only low-risk women with G1 or G2 endometrioid type cancer were included, whereas in the other studies both low-risk and high-risk cases were included. Diagnostic performance of Karlsson's criteria might be overestimated in those studies including high-risk cases. In fact, a study reported by Akbayir et al. [26] found that sensitivity of Karlsson's criteria in G1 or G2 endometrioid carcinomas is (62%) as compared with that in G3 carcinomas (83%). Furthermore, the sample size in those studies reporting high sensitivity was small (67 to 96 cases), which also might contribute to overestimation. Actually, other studies with significantly larger sample size and including high-risk cases, reported poor sensitivity [26].
We observed that the impression of examiner performed better than Karlsson's criteria. A plausible explanation for this is a selection bias, since we only included G1 or G2 tumors. These tumors trend to be small tumors with rather thin endometrium. The result would be a ratio between maximal anteroposterior diameter of the endometrial lesion and the uterine anterior-posterior diameter less than 50%. However, they may invade the myometrium deeply, producing false negative results. Our results for endometrial thickness are also poor in terms of sensitivity. Mascilini et al. [18] reported a sensitivity of 75%. De Smet et al. [15], using a cut-off of endometrial thickness ≥14 mm reported a sensitivity of 81%. Probably, this controversial result might be explained by different selection criteria of patient.
Regarding 3D objective measurements, tumor/uterine 3D volume ratio had a good sensitivity (91%) but very low specificity (28%) in our series. This is in agreement with data reported by Mascilini et al. [18], who reported sensitivity of 75% and specificity of 49%. However, De Smet et al. [15] reported better specificity (80%) but poorer sensitivity (69%). On the other hand, TDS performed poorer than our previous report [17]. Again, in our opinion, these controversial results could be explained by different selection criteria. However, these parameters did not improve the diagnostic performance of subjective impression. Therefore, the role of 3D ultrasound seems to be limited in this setting.
We found that subjective impression performed similarly to subjective model developed by van Holsbeke et al. [16]. The similarity between the two approaches can be explained by the fact that the performance of this subjective model may be heavily affected by the variable subjective assessment. In fact, looking at the formula for this model, the odds ratio for subjective impression (2.3) is about double the odds ratio for preoperative grading (1.1) [16]. Therefore, adding preoperative grade might not significantly increase the model's ability for predicting deep myometrial infiltration as compared with subjective impression only.
In conclusion, the subjective impression of examiner seems to be the best approach for assessing myometrial infiltration in G1 or G2 endometrioid type endometrial cancer by transvaginal or transrectal ultrasound. The use of mathematical models and other objective 2D and 3D measurement techniques do not improve diagnostic performance.
Figures and Tables
Fig. 1
Transvaginal ultrasound showing measurement of tumor/anteroposterior uterine diameter ratio as proposed by Karlsson. In this case the ratio is ≥50% indicating myometrial infiltration of ≥50%.
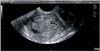
Fig. 2
Transvaginal ultrasound showing measurement of tumor/anteroposterior uterine diameter ratio as proposed by Karlsson. In this case the ratio is <50% indicating myometrial infiltration of <50%.
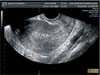
Fig. 3
Three-dimensional ultrasound estimation of tumor (A) and uterine volumes (B). In this case the ratio is 0.118, indicating myometrial infiltration of <50%.
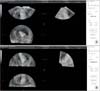
Fig. 4
Three-dimensional ultrasound estimation of shortest tumor distance to serosa (TDS). In this case TDS is 10.5 mm, indicating myometrial infiltration of <50%.
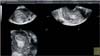
Table 1
Correlation for myometrial infiltration between histology and ultrasound

Table 2
Diagnostic performance of all six approaches for assessing myometrial infiltration

Table 3
Diagnostic performance of all six approaches for assessing myometrial infiltration in those 74 cases with all information available

References
1. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. A Gynecologic Oncology Group Study. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1987; 60:8 Suppl. 2035–2041.
2. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991; 40:55–65.
3. Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, Mariani A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: current evidence. J Obstet Gynaecol Res. 2014; 40:301–311.
4. SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, Leitao M, Salom E, Gehrig P, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol. 2014; 134:385–392.
5. Kumar S, Medeiros F, Dowdy SC, Keeney GL, Bakkum-Gamez JN, Podratz KC, et al. A prospective assessment of the reliability of frozen section to direct intraoperative decision making in endometrial cancer. Gynecol Oncol. 2012; 127:525–531.
6. Cacciatore B, Lehtovirta P, Wahlstrom T, Ylanen K, Ylostalo P. Contribution of vaginal scanning to sonographic evaluation of endometrial cancer invasion. Acta Oncol. 1989; 28:585–588.
7. Cagnazzo G, D'Addario V, Martinelli G, Lastilla G. Depth of myometrial invasion in endometrial cancer: preoperative assessment by transvaginal ultrasonography and magnetic resonance imaging. Ultrasound Obstet Gynecol. 1992; 2:40–43.
8. Osmers RG, Osmers M, Kuhn W. Prognostic value of transvaginal sonography in asymptomatic endometrial cancers. Ultrasound Obstet Gynecol. 1995; 6:103–107.
9. van Doorn HC, van der Zee AG, Peeters PH, Kroeks MV, van Eijkeren MA. Preoperative selection of patients with low-stage endometrial cancer at high risk of pelvic lymph node metastases. Int J Gynecol Cancer. 2002; 12:144–148.
10. Savelli L, Ceccarini M, Ludovisi M, Fruscella E, De Iaco PA, Salizzoni E, et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet Gynecol. 2008; 31:560–566.
11. Antonsen SL, Jensen LN, Loft A, Berthelsen AK, Costa J, Tabor A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer: a multicenter prospective comparative study. Gynecol Oncol. 2013; 128:300–308.
12. Gordon AN, Fleischer AC, Reed GW. Depth of myometrial invasion in endometrial cancer: preoperative assessment by transvaginal ultrasonography. Gynecol Oncol. 1990; 39:321–327.
13. Karlsson B, Norstrom A, Granberg S, Wikland M. The use of endovaginal ultrasound to diagnose invasion of endometrial carcinoma. Ultrasound Obstet Gynecol. 1992; 2:35–39.
14. Fotopoulou C, Sehouli J, Schefold JC, Wolf C, Denkert C, Lichtenegger W, et al. Preoperative transvaginal ultrasound (TVS) in the description of pelvic tumor spread in endometrial cancer: results of a prospective study. Anticancer Res. 2008; 28(4C):2453–2458.
15. De Smet F, De Brabanter J, Van den Bosch T, Pochet N, Amant F, Van Holsbeke C, et al. New models to predict depth of infiltration in endometrial carcinoma based on transvaginal sonography. Ultrasound Obstet Gynecol. 2006; 27:664–671.
16. Van Holsbeke C, Ameye L, Testa AC, Mascilini F, Lindqvist P, Fischerova D, et al. Development and external validation of new ultrasound-based mathematical models for preoperative prediction of high-risk endometrial cancer. Ultrasound Obstet Gynecol. 2014; 43:586–595.
17. Alcazar JL, Galvan R, Albela S, Martinez S, Pahisa J, Jurado M, et al. Assessing myometrial infiltration by endometrial cancer: uterine virtual navigation with three-dimensional US. Radiology. 2009; 250:776–783.
18. Mascilini F, Testa AC, Van Holsbeke C, Ameye L, Timmerman D, Epstein E. Evaluating myometrial and cervical invasion in women with endometrial cancer: comparing subjective assessment with objective measurement techniques. Ultrasound Obstet Gynecol. 2013; 42:353–358.
19. Alcazar JL, Pineda L, Caparros M, Utrilla-Layna J, Juez L, Minguez JA, et al. Transvaginal/transrectal ultrasound for preoperative identification of high-risk cases in well or moderately differentiated endometrioid carcinoma of the endometrium. Ultrasound Obstet Gynecol. 2015; 05. 29. [Epub]. DOI: 10.1002/uog.14912.
20. Lewin SN. Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol. 2011; 54:215–218.
21. Eriksson LS, Lindqvist PG, Floter Radestad A, Dueholm M, Fischerova D, Franchi D, et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol. 2015; 45:476–482.
22. Prompeler HJ, Madjar H, du Bois A, Lattermann U, Wilhelm C, Kommoss F, et al. Transvaginal sonography of myometrial invasion depth in endometrial cancer. Acta Obstet Gynecol Scand. 1994; 73:343–346.
23. Weber G, Merz E, Bahlmann F, Mitze M, Weikel W, Knapstein PG. Assessment of myometrial infiltration and preoperative staging by transvaginal ultrasound in patients with endometrial carcinoma. Ultrasound Obstet Gynecol. 1995; 6:362–367.
24. Gabrielli S, Marabini A, Bevini M, Linsalata I, Falco P, Milano V, et al. Transvaginal sonography vs. hysteroscopy in the preoperative staging of endometrial carcinoma. Ultrasound Obstet Gynecol. 1996; 7:443–446.
25. Sawicki W, Spiewankiewicz B, Stelmachow J, Cendrowski K. The value of ultrasonography in preoperative assessment of selected prognostic factors in endometrial cancer. Eur J Gynaecol Oncol. 2003; 24:293–298.
26. Akbayir O, Corbacioglu A, Numanoglu C, Guleroglu FY, Ulker V, Akyol A, et al. Preoperative assessment of myometrial and cervical invasion in endometrial carcinoma by transvaginal ultrasound. Gynecol Oncol. 2011; 122:600–603.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download