Abstract
Objective
The concept of platinum sensitivity and cross-resistance among platinum agents are widely known in the management of recurrent ovarian cancer. The aim of this study was to evaluate two hypotheses regarding the validity of the concept of platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer.
Methods
In this retrospective study, the clinical data of patients with recurrent cervical cancer, who had a history of receiving cisplatin based chemotherapy (including concurrent chemoradiotherapy [CCRT] with cisplatin) and who received second-line chemotherapy at the time of recurrence between April 2004 and July 2012 were reviewed.
Results
In total, 49 patients-34 squamous cell carcinomas (69.4%) and 15 non-squamous cell carcinomas (30.6%)-were enrolled. The median age was 53 years (range, 26 to 79 years). Univariate and multivariate analysis showed that a platinum free interval (PFI) of 12 months has a strong relationship with the response rate to second-line chemotherapy. Upon multivariate analysis of survival after second-line platinum-based chemotherapy, a PFI of 12 months significantly influenced both progression-free survival (hazard ratio [HR], 0.349; 95% confidence interval [CI], 0.140 to 0.871; p=0.024) and overall survival (HR, 0.322; 95% CI, 0.123 to 0.842; p=0.021). In patients with a PFI of less than 6 months, the difference of progression-free survival between patients with re-administration of cisplatin (3.0 months) and administration of cisplatin analogue (7.2 months) as second-line chemotherapy was statistically significant (p=0.049, log-rank test).
Cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in females worldwide, accounting for 9% (529,800) of new cancer cases and 8% (275,100) of all cancer deaths among females in 2008 [1]. Although several advances in screening, diagnostic, and treatment modalities have been made, the overall prognosis of cervical cancer has not changed dramatically. In cases with distant metastases or recurrences for which curative surgery or radiotherapy is not suitable, chemotherapy is an alternative option. However, the prognosis of these cases generally remains poor with a 1-year survival rate between 15% and 20% [2].
Single-agent cisplatin has been considered the most active agent for advanced or recurrent disease with a response rate of 38% [3]. In 2005, Long et al. [4] reported the results of a randomized phase III trial of cisplatin with or without topotecan (Gynecologic Oncology Group, GOG179). Although this study was the first clinical trial to show a survival advantage of combination therapy over single-agent cisplatin, the response rate and survival after combination therapy were not improved, and single-agent treatment with cisplatin was less efficacious than in historical trials, including other GOG trials [56]. This can be explained by the greater number of patients treated with second-line cisplatin in the GOG179 trial than in other GOG trials owing to the worldwide spread of concurrent chemoradiotherapy (CCRT) with cisplatin. In fact, the number of patients previously treated with cisplatin is rapidly increasing, and in the most recent study, the GOG240 trial, the proportion was about 75% [7].
One of the most important prognostic factors in recurrent ovarian cancer is the platinum free interval (PFI), which is defined as the period between the completion of platinum-based primary chemotherapy and disease recurrence; patients with recurrent ovarian cancer and a PFI of more than 6 months are classified as platinum sensitive [8]. We hypothesized that the PFI would affect the efficacy of second-line platinum-based chemotherapy for patients with recurrent cervical cancer who were previously treated with cisplatin-based chemotherapy and that the concept of 'platinum sensitivity' would be applicable to recurrent cervical cancer.
Kitagawa et al. [9] conducted a randomized phase III trial (Japan Clinical Oncology Group, JCOG0505), and they showed the inferiority of carboplatin/paclitaxel to cisplatin/paclitaxel in terms of progression-free survival (PFS) and overall survival (OS). In this trial, they found a large treatment effect with cisplatin/paclitaxel in patients who had not received prior platinum treatment, and carboplatin/paclitaxel was more effective than cisplatin/paclitaxel for patients with a history of platinum administration. This result suggests that there might be non-cross-resistance of cisplatin analogue with cisplatin. Therefore, the aim of this retrospective study is to evaluate these hypotheses regarding the validity of the concept of 'platinum sensitivity' and 'non-cross-resistance' of cisplatin analogue with cisplatin in recurrent cervical cancer.
We retrospectively reviewed the medical charts of recurrent cervical cancer patients treated between 2002 and 2012 at Shizuoka Cancer Center Hospital. We evaluated the relationship between the PFI and the response to second-line platinum-based chemotherapy, as well as PFS and OS after second-line chemotherapy, and the difference of efficacy between the readministration of cisplatin and the administration of cisplatin analogue as second-line platinum chemotherapy. The response to second-line chemotherapy was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1. The PFI was defined as the period from the completion of first-line platinum-based chemotherapy to the date of diagnosis of recurrence. PFS was measured from the start date of second-line chemotherapy to the date of subsequent radiologic relapse or progression, or to the date of last contact for disease-free patients. OS was defined as the period from the start date of second-line chemotherapy to death or the date of last contact.
To be included in this study, all of the following conditions had to be met: (1) the patients had been treated with cisplatin-based chemotherapy as their initial treatment; (2) the patients had been treated with second-line platinum-based chemotherapy, with the exception of CCRT, which was not regarded as a second-line chemotherapy, even if it included a platinum agent; (3) the patients with performance status of less than 3; (4) histological confirmation of the primary site; (5) cases in which the histologic type was squamous cell carcinoma (SCC), adenocarcinoma (AC), and adenosquamous cell carcinoma (ASC); and (6) measurable disease on radiologic findings. Patients were excluded if second-line platinum-based chemotherapy was administered for fewer than two cycles.
Data regarding the following clinicopathological parameters were recorded for analysis: (1) age at the time of diagnosis of recurrence; (2) histologic type; (3) International Federation of Gynecology and Obstetrics (FIGO) stage at initial diagnosis; (4) information regarding radiation therapy at initial treatment; (5) site of recurrence; (6) second-line chemotherapy regimen; (7) response to second-line chemotherapy assessed according to RECIST ver. 1.1; (8) date of subsequent radiologic relapse or progression; (9) date of death or last contact; and (10) cause of death.
The survival curves were determined by using the Kaplan-Meier method. Factors influencing survival were analyzed using the log-rank test (univariate) and Cox's proportional hazard regression analysis (univariate and multivariate). These analyses were performed using Dr. SPSS II (SPSS Inc., Chicago, IL, USA) statistical software. Contingency table analysis was performed using the chi-square test.
During the study period, 49 patients were identified with recurrent cervical cancer and were treated with second-line platinum-based chemotherapy. Table 1 shows the patient characteristics. The median age was 53 years (range, 26 to 79 years). Thirty-four patients had SCC and 15 had AC or ASC. At initial treatment, most patients (n=36, 73.5%) underwent CCRT with weekly cisplatin. For second-line chemotherapy, 23 patients (46.9%) received cisplatin-based chemotherapy and 26 patients (53.1%) received cisplatin analogue-based (carboplatin or nedaplatin) chemotherapy. The site of recurrence was in the pelvis in 14 patients (28.6%), at distant site in 29 patients (59.2%), and in both in six patients (12.2%). In 31 patients, the maximum recurrent tumor size was within 30 mm, whereas it was more than 30 mm in the remaining 18 patients. The median PFI was 8.2 months (range, 1.4 to 55.8 months).
The response rate of all patients was assessed every two or three cycles and the overall response rate was 18.3% (three patients [6.1%] achieved a complete response and six patients [12.2%] achieved a partial response). By univariate analysis of factors related to the response to second-line chemotherapy, a PFI of 12 months (p=0.047) and prior radiotherapy (yes vs. no, p=0.026) were determined to be statistically significant factors. However, both of these factors did not reach statistical significance in multivariate analysis for the association with the rate of response to second-line chemotherapy (p=0.055 and p=0.057, respectively) (Table 2).
The median follow-up period for OS after second-line platinum chemotherapy was 12.6 months (range, 2.9 to 94.5 months). Upon multivariate analysis of PFS and OS, a PFI of 12 months (hazard ratio [HR], 0.349; 95% confidence interval [CI], 0.140 to 0.871; p=0.024) and tumor diameter (HR, 3.725; 95% CI, 1.724 to 8.047; p=0.001) significantly influenced PFS and a PFI of more than 12 months was significantly associated with an improvement in OS (HR, 0.322; 95% CI, 0.123 to 0.842; p=0.021) (Table 3).
Fig. 1 displays the OS as estimated by the Kaplan-Meier method for patients with a PFI of more than or less than 12 months; the median values were 27.0 and 12.6 months, respectively (p=0.053, log-rank test).
Table 4 shows the relationship between the rate of response to second-line chemotherapy and various factors in the 23 patients with re-administration of cisplatin and the 26 patients with the administration of cisplatin analogue. In the patients re-administered cisplatin, the response rates of those with a PFI of less than 12 months (p=0.006), stages III to IV (p=0.022) and a history of prior radiotherapy (p=0.026) were significantly poor. On the other hand, in the patients administered cisplatin analogue, there were no factors that significantly influenced the response rate. Fig. 2 shows the PFS for patients with a PFI of less than 6 or 12 months who were treated with cisplatin or a cisplatin analogue as second-line chemotherapy. In the patients with a PFI of less than 6 months, the median PFS in patients with the re-administration of cisplatin was 3.0 months, while, in patients with the administration of cisplatin analogue, it was 7.2 months (Fig. 2A). This difference was statistically significant (p=0.049, log-rank test). However, in the patients with a PFI of less than 12 months, there was no difference between the patients with re-administered cisplatin and those administered cisplatin analogue (median PFS: 3.7 months vs. 5.1 months, p=0.531, log-rank test) (Fig. 2B).
The results of this study suggest that the concept of platinum sensitivity could be applied to recurrent cervical cancer and there might be possibility of non-cross-resistance of cisplatin analogue with cisplatin. With regard to the first of these issues, the rate of response to second-line platinum-based chemotherapy was higher in patients with a PFI of more than 12 months than in those with one of less than 12 months (Table 2) and, upon multivariate analysis of PFS and OS, a PFI of more than 12 months was associated with a significant improvement (Table 3). Thus, a PFI of 12 months was suggested as a predictor of response rate and survival after second-line chemotherapy in patients with recurrent cervical cancer initially treated with cisplatin.
Moore et al. [10] reported a retrospective study in which 428 patients with cervical cancer who received a cisplatin-containing chemotherapy combination in previous GOG studies were evaluated to identify prognostic factors for response to cisplatin-based chemotherapy; they showed that a time to recurrence of less than 1 year was one of four adverse parameters. This might imply the validity of the concept of platinum sensitivity.
Tanioka et al. [11] reported the predictive and prognostic value of the PFI for responses to second-line platinum therapy in 65 patients with recurrent cervical cancer; they showed that a PFI of 12 months is an independent predictive factor of tumor response, and a PFI of 6 months is an independent prognostic factor of survival. Matoda et al. [12] retrospectively studied whether the PFI was a predictive indicator of response to second-line platinum-based chemotherapy in recurrent cervical cancer. They reported that a PFI of more than 24 months is the discriminating point between platinum-sensitive and platinum-resistant cervical cancer. In the current study, we showed that a PFI of 12 months might be a predictive and prognostic factor for second-line therapy in this patient population. The reason why each study proposed a different threshold could be their various biases and limited study population.
In the current study, we showed that there is a possibility of non-cross-resistance of cisplatin analogue with cisplatin. The difference of PFS in patients with a PFI of less than 6 months between re-administered cisplatin and those administered cisplatin analogue was significant (Fig. 2).
The difference in the response rate between the readministration of cisplatin and the administration of cisplatin analogue in second-line chemotherapy was not significant (Table 2). However, the response rate upon the re-administration of cisplatin was poorer in patients with a PFI of less than 6 months than in those with one of more than 6 months, as well as in patients with a PFI of less than 12 months than in those with one of more than 12 months; in addition, the response rate upon the administration of cisplatin analogue was not affected by the PFI (Table 4).
This result would suggest that there might be non-cross-resistance of cisplatin analogue with cisplatin and that patients with recurrent cervical cancer and a history of cisplatin administration, especially those with shorter PFI, should be given cisplatin analogue-based chemotherapy as second-line chemotherapy. Kitagawa et al. [9] conducted a randomized phase III trial for the patients with advanced and recurrent cervical cancer, and they reported that carboplatin/paclitaxel is equally effective as cisplatin/paclitaxel in terms of PFS and OS. However, among patients who were not previously treated with cisplatin, carboplatin/paclitaxel therapy resulted in worse OS than cisplatin/paclitaxel treatment (13 months vs. 23 months; HR, 1.57; 95% CI, 1.06 to 2.32), while, among patients previously treated with cisplatin, carboplatin/paclitaxel was superior to cisplatin/paclitaxel in terms of OS (19 months vs. 16 months; HR, 0.69; 95% CI, 0.47 to 1.02). These results suggest that the significant difference in the efficacies of cisplatin and cisplatin analogues for second-line therapy might be caused by previous treatment.
There was a limitations in the current study. This was retrospective study in only one institution, so the number of patients was limited and there was a selection bias.
Despite this limitation, the current study provides further insight into the concept of platinum sensitivity and the possibility of non-cross-resistance of cisplatin analogue with cisplatin. If these concepts are applied to recurrent cervical cancer, patients who were previously treated with cisplatin could obtain useful information about which treatment should be chosen for second-line therapy depending on their PFI and their history of prior platinum administration. In addition, the design of future clinical trials could be influenced by these factors. We advocate that further analyses based on a prospective study should be initiated to confirm these concept of platinum sensitivity and non-cross-resistant of cisplatin analogue with cisplatin.
Figures and Tables
 | Fig. 1Kaplan-Meier curves of overall survival (OS): a platinum free interval (PFI) more than 12 mo vs. less than 12 mo. The median OS: 27.0 mo vs. 12.6 mo, respectively (log-rank test). |
 | Fig. 2Kaplan-Meier curves of platinum free interval (PFI) of a PFI of less than 6 mo: administration of cisplatin (CDDP) analogue vs. readministration of CDDP. (A) The median progression-free survival (PFS) of a PFI of less than 6 mo: 7.2 mo vs. 3.0 mo, respectively (log-rank test). (B) The median PFS of a PFI of less than 12 mo: 5.1 mo vs. 3.7 mo, respectively (log-rank test). |
Table 1
Patient characteristics
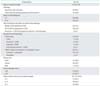
Table 2
Univariate and multivariate analysis for response to second platinum-based chemotherapy
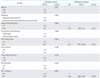
Table 3
Cox proportional hazard analysis on progression-free survival and overall survival

Table 4
The response rate to second-line CDDP based chemotherapy and second-line CDDP analogue based chemotherapy

References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Cadron I, Van Gorp T, Amant F, Leunen K, Neven P, Vergote I. Chemotherapy for recurrent cervical cancer. Gynecol Oncol. 2007; 107:1 Suppl 1. S113–S118.
3. Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing J. Cisplatinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Cancer. 1981; 48:899–903.
4. Long HJ 3rd, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005; 23:4626–4633.
5. Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997; 15:165–171.
6. Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004; 22:3113–3119.
7. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014; 370:734–743.
8. Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991; 9:389–393.
9. Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol. 03. 02. 2015; [Epub]. DOI: 10.1200/JCO.2014.58.4391.
10. Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010; 116:44–49.
11. Tanioka M, Katsumata N, Yonemori K, Kouno T, Shimizu C, Tamura K, et al. Second platinum therapy in patients with uterine cervical cancer previously treated with platinum chemotherapy. Cancer Chemother Pharmacol. 2011; 68:337–342.
12. Matoda M, Tanigawa T, Omatsu K, Ushioda N, Yamamoto A, Okamoto S, et al. Platinum-free interval in second-line chemotherapy for recurrent cervical cancer. Int J Gynecol Cancer. 2013; 23:1670–1674.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download