Abstract
Objective
To assess actual rates of late vaginal stenosis and identify predisposing factors for complications among patients with previously untreated cervical cancer following high-dose-rate brachytherapy.
Methods
We performed longitudinal analyses of 57 patients using the modified Dische score at 6, 12, 18, 24, 36, and 60 months after treatment, which consisted of 15 interstitial brachytherapys and 42 conventional intracavitary brachytherapys, with a median follow-up time of 36 months (range, 6 to 144 months).
Results
More than half of the patients developed grade 1 (mild) vaginal stenosis within the first year of follow-up, and grade 2 (97.5%, moderate) to grade 3 (severe) stenosis gradually increased with time. Actual stenosis rates for grade 1, 2, and 3 were 97.5% (95% confidence interval [CI], 92.7 to 97.5), 60.7% (95% CI, 42.2 to 79.3), and 7.4% (95% CI, 0 to 18.4) at 3 years after treatment. Pallor reaction grade 2-3 at 6 months was only a statistically significant predisposing factor for grade 2-3 late vaginal stenosis 3 years or later with a hazard ratio of 3.48 (95% CI, 1.32 to 9.19; p=0.018) by a multivariate Cox proportional hazard model. Patients with grade 0-1 pallor reaction at 6 months showed a grade ≥2 vaginal stenosis rate of 53%, whereas the grade 2-3 pallor reaction group achieved a grade ≥2 vaginal stenosis rate at 3 years at 100% (p=0.001).
Conclusion
High-dose-rate brachytherapy was associated with high incidence of late vaginal stenosis. Pallor reaction grade 2-3 at 6 months was predictive of late grade 2-3 vaginal stenosis at 3 years after treatment. These findings should prove helpful for patient counseling and preventive intervention.
Radiotherapy plays an important role in the management of cervical cancers [1]. Because the vagina is considered to be relatively resistant to radiation, no serious adverse reaction has particularly been reported [123456]. However, the international EMBRACE study reported that vaginal stenosis was the most frequent complication, with the highest actual probability rates, followed by vaginal dryness after radiotherapy for previously untreated cervical cancer during the first 2 years of follow-up [7]. In addition, we reported a relatively high rate of late vaginal stenosis after high-dose-rate brachytherapy after treatment in various previously untreated or recurrent gynecological cancers, including cervical cancer [56]. The toxicities should not be ignored because they may be potential serious complications [2356]. Although we analyzed the toxicity rate in a previous study, we used crude rates, not considering patients lost to follow-up; thus, actual rates may be higher than reported. Therefore, in the present study, we assessed actual vaginal stenosis rates later than 3 years. To compare our data with those of the EMBRACE study, we selected patients with previously untreated cervical cancer. An additional aim of this study was to identify predisposing factors for late vaginal stenosis because, with regard to the Cochrane study, Miles and Johnson [8] have reported that routine dilation during or immediately after cancer treatment may be harmful, although dilation therapy can be beneficial once the inflammation has resolved. Thus, we identified predictive factors for late vaginal stenosis to help determining the appropriate time to use a vaginal dilator. Because we found a significant correlation between stenosis and pallor reaction [56], we included the incidence of an earlier pallor reaction as a candidate predisposing factor for late vaginal stenosis.
We retrospectively examined 57 patients (median age, 59 years [range, 30 to 88 years]) treated with brachytherapy for previously untreated cervical cancer between 1993 and 2011. The patient characteristics are listed in Table 1. To simplify the examination process, we included only cervical cancer patients who underwent radiotherapy without surgery for previously untreated cervical cancer. The median follow-up was 36 months (range, 6 to 144 months). The patients were divided into two groups: an intracavitary brachytherapy group (n=42) and an interstitial brachytherapy group (n=15). Treatment details are described elsewhere [56]. In brief, an average of 30 Gy (range, 0 to 50.4 Gy) of external irradiation was administered to the entire pelvic field (whole pelvic radiotherapy, WP) with an average of 20 Gy (range, 10 to 40 Gy) to the center-shielded field (CS; entire pelvis plus midline block) over an average period of 4 weeks (range, 2 to 5 weeks). An average of 30 Gy (range, 16.5 to 47 Gy) was administered to point A by intracavitary brachytherapy at an average of 4 fractions (range, 2 to 5 Gy fractions) once a week over an average period of 4 weeks (range, 2 to 7 weeks).
Interstitial brachytherapy was administered at 30 to 36 Gy (6 Gy per fraction, twice per day) [5] at a margin of 5 to 7 mm from the metal marker (superior, inferior, right, left, anterior, and posterior) under the guidance of computed tomography (CT) until May 2005 [9]. After July 2005, we adapted CT-based planning under the guidance of magnetic resonance imaging using a plastic flexible needle applicator as a reference to contour the high-risk clinical target volume (HR CTV) [10]. Thereafter, we administered brachytherapy to the HR CTV covered by the 100% prescribed isodose line. Treatment planning was performed using the PLATO planning system version 14.2 (Nucletron, Veenendaal, the Netherlands) with manual modification after computer optimization.
We used microSelectron-HDR (Nucletron) with 192iridium as the treatment source for brachytherapy. No patient received hormone therapy. We assessed treatment efficacy at 6, 12, and 18 months and 2, 3, and 5 years after radiotherapy using the modified Dische score (Figs. 1, 2) [56]. The original vaginal (vagina and cervix) Dische scores were used to assess bleeding (type and severity) and discharge (frequency and type), as well as the incidence of erythema, ulceration, telangiectasia, and stenosis in the vaginal mucosal tissue according to two or three grades [56]. We introduced additional modifications according to late effects observed in normal tissues as per subjective, objective, management, and analytic scales (LENT SOMA), and pallor of mucosa according to the mucosal reaction from the head and neck scoring system in the original Dische score for meticulous grading [56]. These assessments were conducted by the same physician (KY) throughout the examination period and were later confirmed by a second physician (HY) and a photographic assessment. The biologically equivalent dose was calculated into equivalent 2-Gy fractions (EQD2) using a linear-quadratic model, where α/β=10 for tumors and α/β=3 for organs at risk.
For statistical analyses, actual stenosis and pallor rates were estimated using the Kaplan-Meier method and examined for significance using the log-rank test. Cases with local recurrence were censored at the time of recurrence. The Cox regression proportional hazard model was used for multivariate analysis of the following variables with differences of more than 20% in 3-year stenosis rates: nodal status, lower extension to the vagina, brachytherapy dose, modality (intracavitary or interstitial), and pallor reaction at 6 months. A probability p<0.05 was considered statistically significant.
Vaginal stenosis gradually increased with time. Mild stenosis appeared within the first year of follow-up in more than half of the patients and moderate to severe stenosis gradually increased with time (Fig. 2). Mild (grade 1), moderate (grade 2), and severe (grade 3) stenosis rates were 56.6% (95% confidence interval [CI], 43.3 to 69.0), 7% (95% CI, 0.4 to 13.6), and 0% at 6 months, respectively. These figures decreased to 88.6% (95% CI, 80.0 to 97.1), 20.6% (95% CI, 9.7 to 31.5), and 1% (95% CI, 0 to 5.8) at 1 year; 97.5% (95% CI, 92.7 to 97.8), 60.7% (95% CI, 42.2 to 79.3), and 7.4% (95% CI, 0 to 18.4) at 3 years; and 97.5% (95% CI, 92.7 to 97.8), 75.7% (95% CI, 49.5 to 98.1), and 13.6% (95% CI, 0 to 36.5) at 5 years after treatment, respectively.
The incidence rates of grade ≥2 pallor reaction were 11%, 32%, 68%, and 88% at 6 months and 1, 3, and 5 years after treatment, respectively. Statistically significant correlations were observed between the maximum pallor reaction grade and maximum stenosis grade (p=0.006). Predisposing factors for grade ≥2 late vaginal stenosis are shown in Table 2. Grade ≥2 pallor reaction at 6 months was the only statistically significant predictor for grade ≥2 late vaginal stenosis at 3 and 5 years after treatment. Fifty-three percent of patients with grade 0 or 1 pallor reaction at 6 months showed grade ≥2 stenosis rates at 3 years (69% at 5 years), whereas 100% of patients showed grade ≥2 stenosis rates at 3 years with grade ≥2 pallor reactions at 6 months (p=0.001) (Fig. 3). Patients with grade ≥2 pallor reactions at 6 months (dotted line) showed higher grade ≥2 stenosis rate (100% at 3 years or later) than counterpart (full line, 53% at 3 years and 69% at 5 years; p=0.001).
In addition, brachytherapy dose, a factor and modality (intracavitary vs. interstitial) were found to be predictive factors of developing grade ≥2 stenosis, although these factors showed no statistical significance. The multivariate Cox proportional hazard model revealed that pallor reaction at 6 months was a statistically significant predictive factor for grade ≥2 late vaginal stenosis with a hazard ratio of 3.48 (95% CI, 1.32 to 9.18; p=0.018).
In the recent international prospective EMBRACE study, vaginal stenosis was the most frequent complication, with the highest actual probability rates for both grade ≥1 (75%) and grade ≥2 (22%), followed by vaginal dryness during the first 2 years of follow-up. The prevalence rates of both vaginal stenosis and dryness increased during follow-up [7]. In the 1990s, Bruner et al. [11] have documented a 1.5-cm decrease in vaginal length after intracavitary radiation therapy in patients with cervical or endometrial cancer. In addition, Jensen et al. [12] have found that 48% of patients reported a decrease in vaginal dimensions following radiation for cervical cancer with a 1-year follow-up. In accordance with these results, we found that 20.6% of patients experienced moderate vaginal stenosis after 1 year, which dramatically increased to 73.8% at 5 years, with 16.7% of cases having complete vaginal stenosis. Because grade ≥2 late vaginal stenosis may not only influence the patients' quality of life but also complicate assessment of tumor recurrence, physicians should pay attention to these high incidences of vaginal stenosis.
A vaginal dilator is sometimes used for treatment of vaginal stenosis [1314]. Bahng et al. [14] have reported that with increasing age, the use of a vaginal dilator at least two to three times per week and a shorter active length were found to be significantly associated with a decreased risk of vaginal stenosis. In contrast, with regard to the Cochrane study, Miles and Johnson [8] have reported that routine dilation during or soon after cancer treatment may be harmful. However, there is no reliable evidence to indicate that routine, regular vaginal dilation during or after radiotherapy prevents the late effects of radiotherapy or improves quality of life [8]. Gentle vaginal exploration may separate the vaginal walls before they adhere to one another and some women may benefit from dilation therapy once inflammation has resolved; however, there are no reliable comparative supporting data [8].
Pallor reaction is phenomena related to mucosal thinning and dryness, atrophy, inflammation, and/or fibrosis. Moreover, pallor reaction is reported to be correlated with vaginal stenosis, as in our study [567]. Therefore, we examined and found that that grade ≥2 pallor reaction at 6 months may be a useful predictive factor for grade ≥2 late vaginal stenosis. Early detection and/or preventive intervention of vaginal stenosis, particularly through patient education, or the formulation of some other remedy when the physician recognizes a grade ≥2 pallor reaction, would be fruitful if these observations could prevent vaginal stenosis or relieve symptoms. Therefore, earlier identification of pallor reaction could be a surrogate indicator for late vaginal stenosis and a candidate for dilator intervention.
The recent advanced image-guided brachytherapy observations confirmed that three-dimensional meticulous assessment using dose volume histogram analysis is possible. Fidarova et al. [15] have reported a negative correlation between D2cc in a dose/volume histogram and grade of side effects (telangiectasia and vaginal stenosis). They have speculated that a high dose (D2cc=141 Gy, EQD2) to the upper vagina represents the plateau of the dose-response curve for vaginal adverse events occurring in the area receiving a very high total dose.
There were several limitations in our study that should be addressed. First, this was a single-institute, retrospective analysis of a relatively small patient cohort with a limited follow-up period. Second, the lower extension to the vagina and the prescribed dose by brachytherapy may be important factors that influenced vaginal toxicities, although we found no significant correlation. In addition, we were unable to assess dose-volume histogram data because of the two-dimensional dose prescription. To address these shortcomings, we are awaiting to report of longer-term, prospective results of the international EMBRACE study to obrachytherapyain reliable and reproducible outcomes.
In conclusion, high-dose-rate brachytherapy was associated with a high incidence of late vaginal stenosis. Pallor reaction grade 2-3 at 6 months predicted late vaginal stenosis grade 2-3 at 3 years after treatment. Therefore, pallor reaction at 6 months may be helpful for patient counseling, and preventive intervention.
Figures and Tables
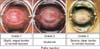 | Fig. 1 |
 | Fig. 3Grade ≥2 actual stenosis rates according to pallor score grade ≥2 at 6 months. Patients with grade ≥2 pallor reactions at 6 months (dotted line) showed higher grade ≥2 stenosis rate (100% at 3 years or later) than counterpart (full line, 53% at 3 years and 69% at 5 years; p=0.001). |
Table 1
Patient characteristics

Table 2
Analysis of prognostic factors for grade ≥2 stenosis after high dose rate brachytherapy
ACKNOWLEDGMENTS
This work was supported by JSPS Grant-in-Aid for Scientific Research (KAKENHI) (C) Grant Number 25461931.
References
1. Barbera L, Thomas G. Management of early and locally advanced cervical cancer. Semin Oncol. 2009; 36:155–169.
2. Gondi V, Bentzen SM, Sklenar KL, Dunn EF, Petereit DG, Tannehill SP, et al. Severe late toxicities following concomitant chemoradiotherapy compared to radiotherapy alone in cervical cancer: an inter-era analysis. Int J Radiat Oncol Biol Phys. 2012; 84:973–982.
3. Teshima T, Inoue T, Ikeda H, Miyata Y, Nishiyama K, Inoue T, et al. High-dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Final results of Osaka University Hospital. Cancer. 1993; 72:2409–2414.
4. Toita T, Kitagawa R, Hamano T, Umayahara K, Hirashima Y, Aoki Y, et al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol. 2012; 126:211–216.
5. Yoshida K, Yamazaki H, Nakamura S, Masui K, Kotsuma T, Baek SJ, et al. Comparisons of late vaginal mucosal reactions between interstitial and conventional intracavitary brachytherapy in patients with gynecological cancer: speculation on the relation between pallor reaction and stenosis. Anticancer Res. 2013; 33:3963–3968.
6. Yoshida K, Yamazaki H, Nakamura S, Masui K, Kotsuma T, Akiyama H, et al. Longitudinal analysis of late vaginal mucosal reactions after high-dose-rate brachytherapy in patients with gynecological cancer. Anticancer Res. 2014; 34:4433–4438.
7. Kirchheiner K, Nout RA, Tanderup K, Lindegaard JC, Westerveld H, Haie-Meder C, et al. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study. Int J Radiat Oncol Biol Phys. 2014; 89:88–95.
8. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2010; (9):CD007291.
9. Yoshida K, Yamazaki H, Takenaka T, Kotsuma T, Yoshida M, Furuya S, et al. A dose-volume analysis of magnetic resonance imaging-aided high-dose-rate image-based interstitial brachytherapy for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2010; 77:765–772.
10. Yamazaki H, Inoue T, Ikeda H, Tang JT, Murayama S, Teshima T, et al. High-dose-rate remote afterloading intestinal radiotherapy employing the template technique for recurrent cancer in the pelvic area. Strahlenther Onkol. 1993; 169:481–485.
11. Bruner DW, Lanciano R, Keegan M, Corn B, Martin E, Hanks GE. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1993; 27:825–830.
12. Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machin D. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2003; 56:937–949.
13. Bakker RM, Vermeer WM, Creutzberg CL, Mens JW, Nout RA, Ter Kuile MM. Qualitative accounts of patients' determinants of vaginal dilator use after pelvic radiotherapy. J Sex Med. 2015; 12:764–773.
14. Bahng AY, Dagan A, Bruner DW, Lin LL. Determination of prognostic factors for vaginal mucosal toxicity associated with intravaginal high-dose rate brachytherapy in patients with endometrial cancer. Int J Radiat Oncol Biol Phys. 2012; 82:667–673.
15. Fidarova EF, Berger D, Schussler S, Dimopoulos J, Kirisits C, Georg P, et al. Dose volume parameter D2cc does not correlate with vaginal side effects in individual patients with cervical cancer treated within a defined treatment protocol with very high brachytherapy doses. Radiother Oncol. 2010; 97:76–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download