1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
2. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95:Suppl 1. S105–S143.
3. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100:1707–1716.
4. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–136.
5. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22:Suppl 6. vi35–vi39.
6. L'Institut National du Cancer (INCa). Cancer de l'endomètre. Collection Recommandations & référentiels. Boulogne-Billancourt: INCa;2010.
7. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105:103–104.
8. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
9. Galaal K, Bryant A, Fisher AD, Al-Khaduri M, Kew F, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2012; 9:CD006655.
10. Lu Q, Liu H, Liu C, Wang S, Li S, Guo S, et al. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a prospective randomized study with 11-year experience. J Cancer Res Clin Oncol. 2013; 139:1853–1859.
11. Tinelli R, Litta P, Meir Y, Surico D, Leo L, Fusco A, et al. Advantages of laparoscopy versus laparotomy in extremely obese women (BMI>35) with early-stage endometrial cancer: a multicenter study. Anticancer Res. 2014; 34:2497–2502.
12. Eltabbakh GH. Effect of surgeon\'s experience on the surgical outcome of laparoscopic surgery for women with endometrial cancer. Gynecol Oncol. 2000; 78:58–61.
13. Holub Z, Jabor A, Bartos P, Hendl J, Urbanek S. Laparoscopic surgery in women with endometrial cancer: the learning curve. Eur J Obstet Gynecol Reprod Biol. 2003; 107:195–200.
14. Dowdy SC, Borah BJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Haas LR, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012; 127:5–10.
15. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000; 182:1506–1519.
16. Ballester M, Dubernard G, Bats AS, Heitz D, Mathevet P, Marret H, et al. Comparison of diagnostic accuracy of frozen section with imprint cytology for intraoperative examination of sentinel lymph node in early-stage endometrial cancer: results of Senti-Endo study. Ann Surg Oncol. 2012; 19:3515–3521.
17. Fersis N, Gruber I, Relakis K, Friedrich M, Becker S, Wallwiener D, et al. Sentinel node identification and intraoperative lymphatic mapping: first results of a pilot study in patients with endometrial cancer. Eur J Gynaecol Oncol. 2004; 25:339–342.
18. Raimond E, Ballester M, Hudry D, Bendifallah S, Darai E, Graesslin O, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: results of a retrospective multicenter study. Gynecol Oncol. 2014; 133:506–511.
19. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010; 375:1165–1172.
20. Chan JK, Kapp DS, Cheung MK, Shin JY, Stieglitz D, Husain A, et al. Prognostic factors and risk of extrauterine metastases in 3867 women with grade 1 endometrioid corpus cancer. Am J Obstet Gynecol. 2008; 198:216.e1–216.e5.
21. Benedetti Panici P, Basile S, Salerno MG, Di Donato V, Marchetti C, Perniola G, et al. Secondary analyses from a randomized clinical trial: age as the key prognostic factor in endometrial carcinoma. Am J Obstet Gynecol. 2014; 210:363.e1–363.e10.
22. Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009; 114:121–127.
23. Goudge C, Bernhard S, Cloven NG, Morris P. The impact of complete surgical staging on adjuvant treatment decisions in endometrial cancer. Gynecol Oncol. 2004; 93:536–539.
24. Ben-Shachar I, Pavelka J, Cohn DE, Copeland LJ, Ramirez N, Manolitsas T, et al. Surgical staging for patients presenting with grade 1 endometrial carcinoma. Obstet Gynecol. 2005; 105:487–493.
25. Frederick PJ, Straughn JM Jr. The role of comprehensive surgical staging in patients with endometrial cancer. Cancer Control. 2009; 16:23–29.
26. Angioli R, Plotti F, Capriglione S, Montera R, Damiani P, Ricciardi R, et al. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol. 2013; 34:571–576.
27. Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012; 13:e353–e361.
28. Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur J Cancer. 2013; 49:3431–3441.
29. Sala P, Morotti M, Menada MV, Cannavino E, Maffeo I, Abete L, et al. Intraoperative frozen section risk assessment accurately tailors the surgical staging in patients affected by early-stage endometrial cancer: the application of 2 different risk algorithms. Int J Gynecol Cancer. 2014; 24:1021–1026.
30. Hahn HS, Song HS, Lee IH, Kim TJ, Lee KH, Shim JU, et al. Magnetic resonance imaging and intraoperative frozen sectioning for the evaluation of risk factors associated with lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2013; 23:1411–1416.
31. Bendifallah S, Canlorbe G, Huguet F, Coutant C, Hudry D, Graesslin O, et al. A risk scoring system to determine recurrence in early-stage type 1 endometrial cancer: a French multicentre study. Ann Surg Oncol. 2014; 21:4239–4245.
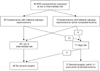
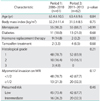







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download