Abstract
Objective
Concurrent chemoradiotherapy (CCRT) is the primary treatment for locally advanced cervical cancer. We studied prognostic factors for patients treated with CCRT.
Methods
We retrospectively reviewed records of 85 consecutive patients with cervical cancer who were treated with CCRT between 2002 and 2011, with external beam radiation therapy, intracavitary brachytherapy, and platinum-based chemotherapy. Survival data were analyzed with Kaplan-Meier methods and Cox proportional hazard models.
Results
Of the 85 patients, 69 patients (81%) had International Federation of Gynecology and Obstetrics (FIGO) stage III/IV disease; 25 patients (29%) had pelvic lymph node enlargement (based on magnetic resonance imaging), and 64 patients (75%) achieved clinical remission following treatment. Median maximum tumor diameter was 5.5 cm. The 3- and 5-year overall survival rates were 60.3% and 55.5%, respectively. Cox regression analysis showed tumor diameter >6 cm (hazard ratio [HR], 2.3; 95% confidence interval [CI], 1.2 to 4.6), pelvic lymph node enlargement (HR, 2.2; 95% CI, 1.1 to 4.5), and distant metastasis (HR, 10.0; 95% CI, 3.7 to 27.0) were significantly and independently related to poor outcomes.
Cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in women, accounting for 8% (275,100) of total cancer deaths among female individuals in 2008 [1]. More than 10 years ago, several randomized controlled trials (RCTs) showed significant survival advantages for patients who received concurrent chemoradiotherapy (CCRT) compared with those who received radiation therapy (RT) alone [2,3,4,5,6]. In Japan, CCRT has also become a standard treatment for locally advanced cervical cancer [7]. However, the introduction of CCRT in Japan differed from that in Western countries. Although RCT populations mostly had International Federation of Gynecology and Obstetrics (FIGO) stage I to II disease [2,3,4,5,6], Japanese patients who actually receive CCRT often have stage III to IVA disease. Because of the nationwide use of Okabayashi radical hysterectomy, patients who present with FIGO stage IB disease rarely receive CCRT; surgery is the treatment of choice for stage IIB disease in Japan [7]. Some Japanese physicians believe that Okabayashi hysterectomy, which corresponds to class IV hysterectomy in Piver's classification, has maximal efficacy, especially against stage IIB disease. The present study reviewed outcomes for all patients with cervical cancer who were treated with CCRT at a single cancer center in Northern Japan between 2002 and 2011, to identify prognostic factors for overall survival (OS). The study population was mainly composed of patients who presented with advanced cervical cancer.
A total of 402 patients with invasive cervical cancer were treated in the National Hospital Organization, Hokkaido Cancer Center from January 2002 to December 2011 (Table 1). Information concerning age, disease stage, histological subtype, treatment, and follow-up was collected by review of the medical records. Of 135 patients who received RT, 92 patients were treated with CCRT. No patients who received RT had previously undergone initial treatment, including surgery. Of the 92 patients, planned RT was not completed in three patients because of worsened physical condition or disease progression. Pretreatment images were not obtained in four patients. Finally, we carried out this study with data from 85 patients with cervical cancer who were treated with CCRT.
Magnetic resonance imaging (MRI) and computed tomography (CT) were performed on all 85 patients before the initial treatment. Acquired images were judged by at least two physicians without knowledge of the clinical findings and survival outcomes. The parameters obtained from MRI were tumor size and pelvic lymph node size. The parameters obtained from CT were distant metastasis. Tumor diameter was measured three dimensionally (anteroposterior, craniocaudal, and horizontal diameters) in either T2-weighted images or gadolinium-enhanced T1-weighted images, which show tumor lesions more clearly. Maximal tumor diameter was used for the present analysis. Lymph node enlargement was defined as a lymph node >10 mm in minimal diameter, as determined radiologically, according to published recommended guidelines [8].
All patients received both external beam radiation therapy (EBRT) and intracavitary brachytherapy (ICBT). EBRT was delivered using 10- or 15-MV X-rays from linear accelerators (Clinac 2100C, Varian, Palo Alto, CA, USA). Whole pelvic irradiation was delivered to a total dose of 50 to 55 Gy in 25 to 27 fractions over 5.5 to 6 weeks with a 4-field box or the anterior-posterior technique. Center shield radiotherapy was performed for shorter overall treatment time to reduce organ-risk exposure, depending on tumor shrinkage after 30.8 to 39.6 Gy had been delivered. ICBT followed the EBRT. All patients underwent high-dose rate brachytherapy, which was delivered to a total dose of 20 Gy in four fractions to the point A over 4 weeks with 192-iridium remote after loading system (RALS; Microselectron, Nucletron, Veenendaal, Netherlands). The point A dose prescription using the Manchester method was performed with the ICBT planning system (Plato@; Nucletron). Image-guided optimization was not applicable even in the case of CT-based ICBT planning. A tandem-cylinder was used only in cases with vaginal involvement of more than one-third of total vaginal length or of an extraordinarily narrow vagina.
As standard chemotherapy, 25 mg/m2 of cisplatin was administered intravenously for 4 days with appropriate hydration and antiemetics, and repeated every 3 weeks for at least two cycles. This schedule was started concomitantly with EBRT. Intravenous administration of 25 mg/m2 of nedaplatin cis-diammine-glycolatoplatinum (CDGP) for 4 days was also used at the discretion of the attending physician. This regimen was also repeated every 3 weeks for at least two cycles. Some patients received systemic chemotherapy before CCRT at the discretion of attending physicians. Indications for use of neoadjuvant chemotherapy were not standardized in our institution.
The primary outcome measure was OS, which was defined as the time from the starting date of initial treatment to death. Patients known to still be alive or lost to follow-up at the time of analysis were censored at their last follow-up. Survival rates were estimated by the Kaplan-Meier method. Cox regression analysis was used to select prognostic factors. Seven variables were used for this analysis; every variable had a binary classification: (1) patient's age (<median vs. ≥median); (2) histological variant (squamous cell carcinoma vs. non-squamous cell carcinoma); (3) maximum tumor diameter (<6 cm vs. ≥6 cm); (4) pelvic lymph node enlargement (no vs. yes); (5) distant metastasis (no vs. yes); (6) implementation of chemotherapy before CCRT (no vs. yes); and (7) pretreatment hemoglobin concentration (<12 g/dL vs. ≥12 g/dL). Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models. The statistical significance level was set at p<0.05. Statistical analyses were performed with StatView-J ver. 5.0 (SAS Institute, Cary, NC, USA).
Table 2 shows the clinicopathological characteristics of the 85 patients included in this study. Their median age was 62 years (range, 34 to 83 years). One patient was in T1b1 (TNM classification), 3 were in T2a, 12 were in T2b, 2 were in T3a, 58 were in T3b, and 9 were in T4a. Seventy-six patients (89%) had squamous cell carcinoma. Eighty-two patients (96%) received cisplatin-based CCRT. No patient who received CDGP-based CCRT also received cisplatin-based chemotherapy. Six patients (7%) had distant metastasis, the breakdown of which was lung 3, liver 1, mediastinal lymph node 1, and lung/liver 1. None of the six patients received systemic chemotherapy before CCRT. Four of the six patients received adjuvant chemotherapy after CCRT, and the remaining two patients received no adjuvant treatment, according to the attending physician's judgment or the patient's wishes.
Twenty-five patients (29%) were radiologically diagnosed with pelvic lymph node enlargement. Median maximum tumor diameter was 5.5 cm. Eight patients (9%) received several courses of systemic chemotherapy before CCRT, including: paclitaxel/cisplatin-4, bleomycin/oncovin/mitomycin/cisplatin-2, and irinotecan/cisplatin-2. Of the eight patients, two were in T2b (tumor sizes: 51, 52 mm), four were in T3 (tumor sizes: 64, 69, 75, 89 mm), and two were inT4 (tumor sizes: 57, 75 mm). Of the eight patients, five had lymph node enlargement but none had distant metastasis at the time of diagnosis.
Sixty-four patients (75%) achieved clinical remission following treatment. Overall, 48 (56%) remained disease free as of their last follow-up visit; 16 (19%) failed treatment (Table 3). The most frequent site of recurrence was the para-aortic nodes, followed by the intrapelvic space.
Table 4 shows the results of Cox regression analysis of prognostic factors in 85 patients. Univariate analysis significantly associated tumor diameter ≥6 cm, pelvic lymph node enlargement and distant metastasis with poorer survival. Low pretreatment hemoglobin levels tended to be associated with poor survival. Multivariate analysis confirmed that tumor diameter ≥6 cm (HR, 2.3; 95% CI, 1.2 to 4.6), pelvic lymph node enlargement (HR, 2.2; 95% CI, 1.1 to 4.5), and distant metastasis (HR, 10.0; 95% CI, 3.7 to 27.0) were significantly and independently related to poor outcomes. Low pretreatment hemoglobin level was not an independent prognostic factor.
Fig. 1 shows the Kaplan-Meier OS curves. The median follow-up period was 32 months overall, and 72 months for patients who did not die of their disease. The 3- and 5-year OS rates were 60.3% and 55.5%, respectively. When the patients are classified into following three groups-(1) patients with tumor diameter <6 cm and no pelvic lymph node enlargement; (2) patients with either tumor diameter ≥6 cm or pelvic lymph node enlargement; and (3) patients with both tumor diameter ≥6 cm and pelvic lymph node enlargement-the three groups significantly differ in OS rates (log-rank test; p=0.001). The 3-year OS rates were 81.2% in those with tumor diameter <6 cm and no pelvic lymph node enlargement, 50.5% in those with either tumor diameter ≥6 cm or pelvic lymph node enlargement, and 23.1% in those with both tumor diameter ≥6 cm and pelvic lymph node enlargement.
Table 5 shows acute and chronic toxicities. No patients died of treatment-related causes. The most common grade 3/4 acute toxicity was neutropenia (27%), followed by diarrhea (15%), anemia (12%), and thrombocytopenia (6%). Nine patients (10.6%) had grade 3/4 late-stage gastrointestinal toxicities. Three patients received a modified plan with extended irradiation field in a caudal direction. One patient received a modified plan with less frequently used center shield. One patient received cisplatin-based CCRT together with paclitaxel. Five patients were surgically treated for ischemic enteritis, or vesicovaginal or rectovaginal fistula.
Previous studies have reported that tumor diameter [9], lymph node enlargement [9,10,11,12], pretreatment hemoglobin level [11,12,13,14], and overall treatment period [15] were confirmed as prognostic factors for patients with cervical cancer who were treated with RT. In the present study, maximum tumor diameter of ≥6 cm, pelvic lymph node enlargement, and distant metastasis are independent prognostic factors.
Of the earlier studies that did not confirm tumor size as an independent risk factor, more than 70% were composed of patients with stage I/II disease [10,11,12]. In such populations, the effect of tumor diameter on survival must be weak because the local tumor volume is usually within the therapeutic range of radiotherapy. In contrast, the effect of tumor size must be stronger in populations mainly composed of patients with stage III/IV disease because tumor size is often beyond the therapeutic limit of radiotherapy. Kodaira et al. [9] demonstrated that maximum tumor diameter of ≥5 cm and lymph node enlargement were independent prognostic factors. However, as eligible patients had stage II disease in that study, the therapeutic limit of CCRT cannot be confirmed in such a population. Our study failed to show prognostic significance for tumors ≥5 cm (HR, 1.7; 95% CI, 0.9 to 3.5) in univariate analysis. On the other hand, tumors 6 to 7 cm had an HR of 2.32 (95% CI, 1.21 to 4.43) and 2.53 (95% CI,-1.19 to 5.38) in univariate analysis and an HR of 2.01 (95% CI, 1.04 to 3.89) and 1.95 (95% CI, 0.89 to 4.30) after adjusting for pelvic lymph node enlargement. Cutoff values for tumor size as a prognostic factor are larger in studies composed mainly of advanced disease than in those composed mainly of early-stage disease. The therapeutic limit of CCRT must therefore be confirmed separately for late-stage and early-stage disease. Tumors 6 to 7 cm in size seem to be a plausible therapeutic limit for CCRT.
Previous studies have associated low pretreatment hemoglobin levels and poorer outcome in cervical cancer patients managed with definitive RT [11,12,13,14]. Fyles et al. [16] presented a review article in 2000 that described anemia as associated with inferior treatment outcome in cervical cancer, whereas hemoglobin levels before and during treatment were strongly correlated with tumor size; which may explain the prognostic effect of anemia in older studies. They concluded that the relationships among anemia and treatment outcome are complex and further study of anemia as an independent prognostic factor is required. In our study, low pretreatment hemoglobin levels had a HR of 2.0 (95% CI, 0.97 to 4.0) in univariate analysis but a decreased HR of 1.4 (95% CI, 0.6 to 3.2) after adjusting tumor diameter. In 2004, a retrospective review of 494 patients with cervical cancer who were treated with CCRT on two consecutive prospective Gynecologic Oncology Group trials was conducted. The study showed that the hemoglobin levels during the late period were most predictive of disease progression [17]. In 2006, Choi et al. [18] reported that hemoglobin levels during CCRT were an independent prognostic factor in patients not found to have lymph node metastasis by MRI. Maintenance of hemoglobin level might not be of benefit in cervical cancer with lymph node metastasis. In 2012, Kuroda et al. [19] conducted a retrospective review of 131 patients with FIGO stage IIIB cervical cancer treated with definitive RT. They did not find pretreatment hemoglobin levels to affect survival outcome, even in univariate analysis [19]. As the study population included many more patients with lymph node metastasis than those reported in Western countries, a sufficient hemoglobin level might have been of no benefit in that study. The earlier studies that associated low pretreatment hemoglobin with poorer outcome had study populations composed mainly or evenly of stage I/II disease [11,12,13]. In our study, pretreatment hemoglobin level and survival outcome were not associated, possibly because of a high percentage of subjects with FIGO stage III/IV disease.
Should patients with tumors of ≥6 cm in diameter and radiologically enlarged pelvic lymph nodes receive CCRT? Systemic chemotherapy before definitive local treatment might improve local control and prognosis for patients with chemosensitive cervical cancer, despite not improving prognoses for all patients with locally advanced cervical cancer. Of eight patients who received systemic chemotherapy before CCRT, five achieved long-term survival, with a range of 57 to 123 months. Of the five patients, two were in T2b (tumor sizes, 51-52 mm) with lymph node enlargement, one was in T3a (tumor size, 75 mm) with pelvic and para-aortic lymph node enlargement, one was in T3b (tumor size, 89 mm), and one was inT4a (tumor size, 57 mm). Although the clinical benefit of systemic chemotherapy before CCRT was not shown in this study, such a benefit might be evident in a future study with many more patients who undergo neoadjuvant chemotherapy.
Of course, our study has some limitations. First, the number of patients included in the study was too small to power conclusive results. Second, concurrent chemotherapy used in our CCRT differed from the worldwide standard regimen (weekly intravenous cisplatin, 40 mg/m2 for five to six cycles). As the standard international regimen is mostly based on studies of Western patients, who tend to be physically larger than Japanese patients, severe toxicity might be a greater problem with Japanese patients. However, our study had long followup periods (median, >70 months) and relatively few cases (8%) were excluded. Based on the higher percentage of FIGO stage III/IV disease in our study population, the present study might give results that differ from those of previous studies in Western countries.
In conclusion, tumor diameter ≥6 cm, radiologically enlarged pelvic lymph node, and distant metastasis were independent prognostic factors for patients with CCRT-treated cervical cancer in a Japanese cohort.
Figures and Tables
Fig. 1
(A) Univariate survival plot by Kaplan-Meier for estimation of overall survival in 85 patients with cervical cancer who were treated with concurrent chemoradiotherapy. (B) Another Kaplan-Meier analysis showed significant differences in overall survival rates among three groups (log-rank test: p=0.001): (a) a group of patients with tumor diameter <6 cm and no pelvic lymph node enlargement; (b) a group of patients with either tumor diameter ≥6 cm or pelvic lymph node enlargement; (c) a group of patients with both tumor diameter ≥6 cm and pelvic lymph node enlargement.

Table 1
Distribution of initial treatment for 402 patients with cervical cancer at the Hokkaido Cancer Center between 2002 and 2011
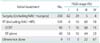
Table 2
Clinical characteristics of patients with cervical cancer treated with CCRT (n=84)

Table 3
Initial failure patterns and details of initial failure sites in cervical cancer patients treated with concurrent chemoradiotherapy
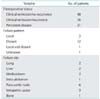
Table 4
Prognostic factors for overall survival rates selected by Cox proportional hazard model analysis

Table 5
Acute toxicity and late complications (NCI-CTC ver. 4.0)

References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999; 17:1339–1348.
3. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340:1144–1153.
4. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999; 340:1137–1143.
5. Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002; 20:966–972.
6. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004; 22:872–880.
7. Nagase S, Inoue Y, Umesaki N, Aoki D, Ueda M, Sakamoto H, et al. Evidence-based guidelines for treatment of cervical cancer in Japan: Japan Society of Gynecologic Oncology (JSGO) 2007 edition. Int J Clin Oncol. 2010; 15:117–124.
8. Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994; 190:807–811.
9. Kodaira T, Fuwa N, Kamata M, Furutani K, Kuzuya K, Ogawa K, et al. Clinical assessment by MRI for patients with stage II cervical carcinoma treated by radiation alone in multicenter analysis: are all patients with stage II disease suitable candidates for chemoradiotherapy? Int J Radiat Oncol Biol Phys. 2002; 52:627–636.
10. Atahan IL, Onal C, Ozyar E, Yiliz F, Selek U, Kose F. Long-term outcome and prognostic factors in patients with cervical carcinoma: a retrospective study. Int J Gynecol Cancer. 2007; 17:833–842.
11. Parker K, Gallop-Evans E, Hanna L, Adams M. Five years' experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institution. Int J Radiat Oncol Biol Phys. 2009; 74:140–146.
12. Lim A, Sia S. Outcomes of chemoradiotherapy in cervical cancer: the Western Australian experience. Int J Radiat Oncol Biol Phys. 2012; 82:1431–1438.
13. Serkies K, Badzio A, Jassem J. Clinical relevance of hemoglobin level in cervical cancer patients administered definitive radiotherapy. Acta Oncol. 2006; 45:695–701.
14. Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999; 86:1528–1536.
15. Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1995; 32:1275–1288.
16. Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000; 57:13–19.
17. Winter WE 3rd, Maxwell GL, Tian C, Sobel E, Rose GS, Thomas G, et al. Association of hemoglobin level with survival in cervical carcinoma patients treated with concurrent cisplatin and radiotherapy: a Gynecologic Oncology Group Study. Gynecol Oncol. 2004; 94:495–501.
18. Choi YS, Yi CM, Sin JI, Ye GW, Shin IH, Lee TS. Impact of hemoglobin on survival of cervical carcinoma patients treated with concurrent chemoradiotherapy is dependent on lymph node metastasis findings by magnetic resonance imaging. Int J Gynecol Cancer. 2006; 16:1846–1854.
19. Kuroda Y, Murakami N, Morota M, Sekii S, Takahashi K, Inaba K, et al. Impact of concurrent chemotherapy on definitive radiotherapy for women with FIGO IIIb cervical cancer. J Radiat Res. 2012; 53:588–593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download