Abstract
Objective
To evaluate the prognostic implication of human papillomavirus (HPV) viral load in cervical cancer patients who underwent radical hysterectomy.
Methods
We conducted a retrospective review of patients with stage IA2 through stage IIIA cervical carcinoma who underwent radical hysterectomy at Sun Yat-sen University Cancer Center between January 2005 and December 2009. Patients who had undergone preoperative hybrid capture 2 testing to detect HPV DNA were included. A total of 346 patients positive for HPV DNA were enrolled and stratified into two groups according to the median HPV viral load.
Results
HPV viral load was significantly correlated with lymphovascular space invasion (p=0.026) and deep stromal invasion (p=0.024). However, other factors, such as age, stage, histologic grade, histologic type, lymph node metastasis, and tumor size, were not significantly associated with viral load. Low HPV viral load was correlated with poor disease-free survival in univariate analysis (p=0.037) and multivariate analysis (p=0.027). There was no significant difference in overall survival with regard to initial HPV viral load.
The role of high-risk human papillomavirus (HPV) in the etiology of cervical cancer has been well established. HPV testing is becoming an integral component of cervical cancer screening. Multiple methods with various sensitivities and specificities have been proposed for detecting HPV in cervical samples [1]. Among them, the hybrid capture 2 (HC2) test is one of the most widely used methods.
Several studies using HC2 have reported that an increased HPV viral load correlates with the severity, progression and recurrence of cervical lesions [2,3,4,5,6,7], and a few studies have revealed that the initial HPV viral load measured by the HC2 test is associated with the prognosis of cervical cancer patients treated with primary radiotherapy [8,9,10]. Among these studies, a prospective observational study of 169 radiotherapy patients [8] has suggested that a low initial HPV viral load is a strong independent prognostic factor associated with higher overall relapse rates.
The initial HPV viral load may be an important predictor of the outcome of radiotherapy in patients with invasive uterine cervical cancer; however, it is unclear whether the initial HPV viral load is an important prognostic factor for patients with surgically treated cervical carcinoma. The purpose of this study was to evaluate the prognostic implication of preoperative HPV viral load in patients with cervical carcinoma treated with radical hysterectomy.
A retrospective review of patients with stage IA2 through stage IIIA cervical carcinoma (International Federation of Gynecology and Obstetrics [FIGO]) who underwent radical hysterectomy and pelvic lymphadenectomy at Sun Yatsen University Cancer Center between January 2005 and December 2009 was conducted. Patients were included only if they had undergone a preoperative HC2 test to detect HPV DNA. We reviewed the clinical records of 500 cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy, 68 patients with no HPV DNA test on record or HPV DNA-negative results were excluded. Among the remaining 432 patients, 34 patients were excluded from the study due to loss to follow-up, 52 of 124 patients who received the neoadjuvant treatments were excluded, because they had no initial record of HPV DNA test before neoadjuvant treatments. Ultimately, 346 cervical cancer patients who underwent radical hysterectomy and pelvic lymphadenectomy with HPV DNA-positive results were enrolled in this study.
A total of 72 patients (20.8%) received chemotherapy or radiation therapy before undergoing radical hysterectomy and pelvic lymphadenectomy. For these patients, the HPV DNA test was performed before the neoadjuvant treatments, not before the surgery. Furthermore, postoperative adjuvant radiation therapy, chemotherapy, or concurrent chemoradiation was performed in patients with risk factors such as lymph node metastasis, parametrial invasion, deep stromal invasion (>two-thirds), lymphovascular space invasion (LVSI), large tumor size (>4 cm), positive or close resection margins. In 346 patients, 248 patients (71.7%) received adjuvant therapy after surgery. 44 of 248 patients (17.7%) received radiation therapy alone, 81 of 248 patients (32.7%) received chemotherapy alone, and 123 of 248 patients (49.6%) received concurrent or sequential chemoradiation.
Post-treatment surveillance consisted of follow-up visits every 3 months for 2 years, every 6 months between years 3 and 5, and yearly after 5 years. During the post-treatment surveillance, each visit consisted of a clinical history assessment, physical and pelvic examinations, ultrasonography of the abdomen and pelvis and serum tumor marker measurement. Chest/abdominal/pelvic computed tomography and magnetic resonance imaging were performed as clinically indicated.
Disease-free survival (DFS) was defined as the interval between the date of primary surgery and the date of recurrence, and overall survival (OS) was defined as the interval between the date of primary surgery and the date of death.
Before surgery, cervical cells for HPV DNA testing were collected by placing a cytobrush into the exocervix and rotating the brush three times. The brush and the collected cellular material were then placed into a vial of Preserv-Cyt solution (Digene, Gaithersburg, MD, USA) before testing.
The HPV DNA titer was measured using the Hybrid Capture 2 High-Risk HPV DNA Test (HC2 test, Digene). The HC2 test is an in vitro nucleic acid hybridization assay; it uses signal amplification with microplate chemiluminescence to detect HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 in cervical specimens. In this assay, luminescence is measured with a luminometer and expressed as relative light units (RLUs). The RLU/cutoff (RLU/CO) ratio is defined as the ratio of specimen luminescence to the luminescence of the 1.0 pg/mL HPV 16 cutoff standard. An RLU/CO ≥1.0 was considered positive.
Demographic and clinical characteristics are presented as counts and percentages for the categorical variables; medians and ranges are provided for the continuous variables. Pearson chi-square tests were performed to assess the relationships between viral load and clinical characteristics in the two groups. Kaplan-Meier survival curves were compared using the log-rank test to determine DFS and OS. Multivariate analysis was performed using the Cox regression method. Hazard ratios (HRs) with their 95% confidence intervals (CIs) are reported. Statistical calculations were performed using the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). All reported pvalues are two-sided. p-values less than 0.05 were considered to be statistically significant.
The patient characteristics are listed in Table 1. The median HPV viral load was 132.5 RLU/CO (range, 1.1 to 3,318.3). The median age was 44 years (range, 22 to 86 years). The FIGO stage was divided into three groups for analysis: IA2/IB, IIA/IIB and IIIA. Of the 346 patients, 250 patients (72.3%) had stage IA2/IB, 95 patients (27.4%) had stage IIA/IIB, and 1 patient (0.3%) had stage IIIA cervical cancer. The median duration of follow-up for all patients was 48.1 months. A total of 32 patients (9.2%) experienced disease recurrence, and 15 patients (4.3%) died as a result of the disease.
The patients were classified into a low or high viral load group according to the median value of 132.5 RLU/CO. HPV viral load was correlated with several clinicopathologic parameters. Tumors with higher viral load had LVSI more frequently than those with lower viral load (12.7% vs.5.8%; p=0.026). Tumors with higher viral load had deep stromal invasion (>two-thirds) more frequently than those with lower viral load (54.9% vs. 42.8%; p=0.024); however, age, FIGO stage, histologic grade, histologic type, lymph node metastasis, and tumor size were not associated with viral load (Table 2).
Low viral load was associated with poor DFS in univariate analysis (p=0.037), no statistically significant difference in OS (p=0.220) were observed between the low and high viral load groups (Fig. 1). Other prognostic parameters for DFS included lymph node metastasis (p<0.001), histologic type (p<0.001), parametrial involvement (p=0.026), and depth of stromal invasion (p=0.018). However, histologic grade, LVSI, FIGO stage, vaginal resection margin positive and tumor size which also assessed for an impact on survival were not significantly associated with DFS in univariate analysis.
In multivariate analysis, low HPV viral load showed significant relationship with poor DFS (HR, 2.39; 95% CI, 1.11 to 5.16; p=0.027). Multivariate analysis also identified lymph node metastasis (HR, 4.42; 95% CI, 1.99 to 9.81; p<0.001), and specific histologic type (neuroendocrine carcinoma and sarcomatoid carcinoma) (HR, 32.62; 95% CI, 8.55 to 124.42; p<0.001) as significant poor prognostic factors for DFS, respectively. These analyses are summarized in Table 3.
In this study, we found that low HPV viral load was associated with poor DFS in patients with surgically treated cervical carcinoma both in univariate analysis and multivariate analysis. Once a patient is diagnosed with cervical cancer, the preoperative detection of HPV with the HC2 test may be necessary, because low initial HPV viral load maybe a poor predictor of cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy.
However, the relationship between low HPV viral load and poor OS was not statistically significant, we give possible reasons as follows: the population of the study was not large enough, the duration of follow-up was not quite long and the limit of retrospective nature of current study. The role of low HPV viral load in treatment failure of cervical cancer need more evidences and studies to verify. Our results indicated that low HPV viral load may be associated with poor outcomes of surgically treated cervical cancer patients. We explain our results as follows. The replication of HPV as an episome leads to a large increase in viral DNA copy number [11]; however, in cervical cancer, HPV often replicates as an extrachromosomal DNA inside the nucleus of the host cell [12], integration of the viral genome disrupts the E1 and E2 genes, therefore the transcriptional repression of early genes is lost [13], results in increased expression of the E6 and E7 oncoproteins [14], although the viral life cycle terminate as large portions of the genome are disrupted and the actual viral DNA copy number is reduced [12]. Furthermore, several studies have reported that E6 and E7 oncoproteins are retained and expressed in cervical tumors and cell lines after viral replication has ceased [15,16,17]. Previous work has shown that increased expression of viral E6 and E7 can contribute to enhanced chemotherapy and radiation therapy resistance [18,19]. In 346 patients of our study, most patients (71.7%) received adjuvant chemotherapy or radiation therapy after surgery. Thus, low HPV viral load indicates the resistance of chemotherapy and radiation therapy, consistent with our result that low viral load maybe associated with poor outcome after comprehensive therapy based on operation.
We also observed that high HPV viral loads were associated with LVSI and deep stromal invasion, which have been previously reported to be correlated with poor prognosis [20,21,22,23]. However, this result seems to conflict with our finding that high HPV viral load was associated with improved DFS. Few previous studies have reported the correlations of pretreatment HPV viral load with clinicopathologic characteristics and prognosis in cervical cancer patients. Kim et al. [8] performed a prospective study that recruited 169 cervical cancer patients treated with radiation therapy. The researchers classified their patients into low- or high-HPV viral load groups based on a median value of 385.8 RLU/CO. The study found that HPV viral load was associated with age, histologic type, and lymph node metastasis. Additionally, low HPV viral load was associated with poor radiotherapy outcome. Kim et al. [24] reviewed 34 early-stage cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy and placed the patients in arbitrary groups based on HPV viral load at 100 RLU/CO. The researchers reported that HPV viral load was not associated with any clinicopathologic characteristics or with prognosis. Consequently, the correlations of pretreatment HPV viral load with clinicopathologic characteristics and prognosis in cervical cancer patients reported in previous studies were inconsistent, the association of pretreatment HPV viral load with other prognostic factors is currently unclear.
In addition, both depth of stromal invasion and LVSI are the poor prognosis factors, and they are also the indicators of adjuvant chemotherapy and/or radiation therapy after surgery. We observed that high HPV viral load group had higher proportions of poor prognostic factors which indicate higher proportions of receiving adjuvant therapy after surgery. Moreover, we have hypothesized that high HPV viral load also associated with the sensitivity of chemotherapy and radiation therapy, it means that patients with higher initial HPV viral load may be more likely to receive adjuvant therapy after surgery, and they're also more sensitive to chemotherapy and radiation therapy; thus, patients with higher initial HPV viral load may have better outcome.
Compared with other studies, our patient population is the largest. However, the current study has several limitations. The HC2 assays did not adjust for cellularity or multiple infections, and median values and ranges vary among laboratories depending on the specimen collection method and experimental conditions, although HC2 is commercially available with U.S. Food and Drug Administration approval and has been demonstrated to be reliable and reproducible [25,26,27,28,29].
In conclusion, low HPV viral load may be a poor prognostic factor for cervical cancer patients who underwent radical hysterectomy and pelvic lymphadenectomy. However, this review had limited power because of its retrospective nature. A future large-scale, prospective study may provide clearer answers.
Figures and Tables
Fig. 1
(A) Disease-free survival and (B) overall survival according to human papillomavirus (HPV) load in 346 patients.

Table 1
Characteristics of patients (n=346)
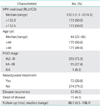
Table 2
Association between human papillomavirus viral load and clinicopathologic characteristics

Table 3
Multivariate analysis for disease-free survival with clinicopathologic prognostic factors
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (81172484/H1621).
References
1. Sandri MT, Lentati P, Benini E, Dell'Orto P, Zorzino L, Carozzi FM, et al. Comparison of the Digene HC2 assay and the Roche AMPLICOR human papillomavirus (HPV) test for detection of high-risk HPV genotypes in cervical samples. J Clin Microbiol. 2006; 44:2141–2146.
2. Origoni M, Carminati G, Rolla S, Clementi M, Sideri M, Sandri MT, et al. Human papillomavirus viral load expressed as relative light units (RLU) correlates with the presence and grade of preneoplastic lesions of the uterine cervix in atypical squamous cells of undetermined significance (ASCUS) cytology. Eur J Clin Microbiol Infect Dis. 2012; 31:2401–2406.
3. Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008; 108:549–554.
4. Wu Y, Chen Y, Li L, Yu G, Zhang Y, He Y. Associations of high-risk HPV types and viral load with cervical cancer in China. J Clin Virol. 2006; 35:264–269.
5. Ho CM, Cheng WF, Chu TY, Chen CA, Chuang MH, Chang SF, et al. Human papillomaviral load changes in low-grade squamous intraepithelial lesions of the uterine cervix. Br J Cancer. 2006; 95:1384–1389.
6. Hernandez-Hernandez DM, Ornelas-Bernal L, Guido-Jimenez M, Apresa-Garcia T, Alvarado-Cabrero I, Salcedo-Vargas M, et al. Association between high-risk human papillomavirus DNA load and precursor lesions of cervical cancer in Mexican women. Gynecol Oncol. 2003; 90:310–317.
7. Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003; 106:396–403.
8. Kim JY, Park S, Nam BH, Roh JW, Lee CH, Kim YH, et al. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol. 2009; 27:5088–5093.
9. Song YJ, Kim JY, Lee SK, Lim HS, Lim MC, Seo SS, et al. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int J Cancer. 2011; 129:896–902.
10. Datta NR, Kumar P, Singh S, Gupta D, Srivastava A, Dhole TN. Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix? A pilot study. Gynecol Oncol. 2006; 103:100–105.
11. Doorbar J. Papillomavirus life cycle organization and biomarker selection. Dis Markers. 2007; 23:297–313.
12. Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci. 2009; 34:113–123.
13. Finzer P, Aguilar-Lemarroy A, Rosl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002; 188:15–24.
14. Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995; 92:1654–1658.
15. Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986; 83:4680–4684.
16. Androphy EJ, Hubbert NL, Schiller JT, Lowy DR. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987; 6:989–992.
17. Banks L, Spence P, Androphy E, Hubbert N, Matlashewski G, Murray A, et al. Identification of human papillomavirus type 18 E6 polypeptide in cells derived from human cervical carcinomas. J Gen Virol. 1987; 68(Pt 5):1351–1359.
18. Hampson L, El Hady ES, Moore JV, Kitchener H, Hampson IN. The HPV16 E6 and E7 proteins and the radiation resistance of cervical carcinoma. FASEB J. 2001; 15:1445–1447.
19. Zheng Y, Zhang J, Rao Z. Ribozyme targeting HPV16 E6E7 transcripts in cervical cancer cells suppresses cell growth and sensitizes cells to chemotherapy and radiotherapy. Cancer Biol Ther. 2004; 3:1129–1134.
20. Waggoner SE. Cervical cancer. Lancet. 2003; 361:2217–2225.
21. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000; 70:209–262.
22. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–540.
23. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990; 38:352–357.
24. Kim YM, Park JY, Lee KM, Kong TW, Yoo SC, Kim WY, et al. Does pretreatment HPV viral load correlate with prognosis in patients with early stage cervical carcinoma? J Gynecol Oncol. 2008; 19:113–116.
25. Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003; 127:940–945.
26. Bolick DR, Bolick RE, Coates F, Daniels CM, Juretich MB, Lin KK, et al. Laboratory implementation of human papillomavirus testing. Arch Pathol Lab Med. 2003; 127:984–990.
27. Castle PE, Lorincz AT, Mielzynska-Lohnas I, Scott DR, Glass AG, Sherman ME, et al. Results of human papillomavirus DNA testing with the hybrid capture 2 assay are reproducible. J Clin Microbiol. 2002; 40:1088–1090.
28. Castle PE, Wheeler CM, Solomon D, Schiffman M, Peyton CL. ALTS Group. Interlaboratory reliability of hybrid capture 2. Am J Clin Pathol. 2004; 122:238–245.
29. Clavel C, Masure M, Levert M, Putaud I, Mangeonjean C, Lorenzato M, et al. Human papillomavirus detection by the hybrid capture II assay: a reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn Mol Pathol. 2000; 9:145–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download