Abstract
Objective
Compression of the left common iliac vein between the right common iliac artery and the vertebrae is known to be associated with the occurrence of left iliofemoral deep vein thrombosis (DVT). In this study, we described the variability in vascular anatomy of the common iliac veins and evaluated the relationship between the degree of iliac vein compression and the presence of DVT using the data from surgeries for gynecologic cancer.
Methods
The anatomical variations and the degrees of iliac vein compression were determined in 119 patients who underwent systematic para-aortic and pelvic lymphadenectomy during surgery for primary gynecologic cancer. Their medical records were reviewed with respect to patient-, disease-, and surgery-related data.
Results
The degrees of common iliac vein compression were classified into three grades: grade A (n=28, 23.5%), with a calculated percentage of 0%-25% compression; grade B (n=47, 39.5%), with a calculated percentage of 26%-50% compression; and grade C (n=44, 37%), with a calculated percentage of more than 50% compression. Seven patients (5.9%) had common iliac veins with anomalous anatomies; three were divided into small caliber vessels, two with a flattened structure, and two had double inferior vena cavae. The presence of DVT was associated with the elevated D-dimer levels but not with the degree of iliac vein compression in this series.
Conclusion
Although severe compression of the common iliac veins was frequently observed, the degree of compression might not be associated with DVT in surgical patients with gynecologic cancer. Anomalous anatomies of common iliac veins should be considered during systematic para-aortic and pelvic lymphadenectomy in the gynecologic cancer patients.
The para-aortic and pelvic lymph nodes are common metastatic sites in gynecologic cancer. Consequently, a systematic para-aortic and pelvic lymphadenectomy is recommended for accurate staging and for planning adjuvant treatment. Evaluation of the para-aortic and pelvic lymph nodes is the standard procedure for early-stage ovarian cancer [1,2]. Although two randomized clinical trials demonstrated that pelvic lymphadenectomy does not improve patient survival rates in endometrial cancer [3,4], a cohort study recently reported that systematic para-aortic and pelvic lymphadenectomy has a therapeutic benefit in patients with intermediate-or high-risk endometrial cancer [5]. Para-aortic and pelvic lymphadenectomy procedures for gynecologic cancer have been well established. However, these procedures require operating in close proximity to multiple vascular structures, and there is always the risk of intraoperative hemorrhage. The potential for vascular trauma can be minimized by carefully defining the vascular anatomy of the region prior to surgery [6].
Compression of the left common iliac vein between the right common iliac artery and the vertebrae is known to be associated with the occurrence of left iliofemoral deep vein thrombosis (DVT) [7,8]. Such compression occasionally results in the development of a spur within the common iliac vein wall. However, whether the degree of iliac vein compression is correlated with incidence of DVT was still uncertain. Most studies on the vascular anatomy of the iliac veins have been conducted using cadavers. As we have gained more experience in para-aortic and pelvic lymphadenectomy, we have noticed anatomical variations in the common iliac veins in patients with gynecologic cancer. In this study, we described the variability in vascular anatomy of the common iliac veins and evaluated the relationship between the degree of iliac vein compression and the presence of DVT in a series of patients who underwent para-aortic and pelvic lymphadenectomy in our institution.
All consecutive patients undergoing systematic para-aortic and pelvic lymphadenectomy during surgery for primary gynecologic cancer at Chiba University Hospital between August 2008 and March 2012 were evaluated for inclusion in this study. Medical records regarding patient-related, diseaserelated, and surgery-related data were retrospectively reviewed. The study protocol was approved by the Institutional Review Board of Chiba University School of Medicine (Chiba University School of Medicine #1163).
A para-aortic lymphadenectomy up to the left renal vein was performed in all patients included in this study. The common iliac arteries were isolated and underrun with marker tape at the site where the common iliac artery crossed over the common iliac vein. The common iliac arteries were lifted upward and the common iliac veins were isolated. Subsequently, presacral lymphadenectomy was performed at the crossing site of the common iliac artery over the common iliac vein. We used an electrocautery and bipolar scissors for lymphadenectomy. After the iliac arteries and veins were stripped of lymphatic-bearing tissue, length of the compressed common iliac vein and the diameter of the common iliac artery at the site where it crosses over the common iliac vein were measured using a vernier caliper (Fig. 1). The diameter of the common iliac vein distal to the crossing site was also measured. We recorded the anatomy of the iliac arteries and veins using videos and photographs in most cases. The anatomical variation and the degree of iliac vein compression were recorded for each patient immediately after surgery. The degree of iliac vein compression was calculated as indicated in Fig. 1C. Because the degree of venous compression was estimated using the calculation from the postsurgical appearance of the iliac vein, we dealt with it as a categorical variable. We referred to the analysis in the previous study and classified the degree of iliac vein compression into three grades: grade A (≤25% compression), grade B (26%-50%), grade C (>50%). In that study, degrees of iliac vein compression with a calculated percentage of more than 50% were classified into the most severe grade [9]. Subsequently, we divided the residual degrees of iliac vein compression in half.
An enhanced computed tomography (CT) scan from the chest to the lower legs was performed to detect the presence of thrombi in patients with elevated D-dimer levels >1.0 µg/mL. If the administration of contrast material was contraindicated, venous ultrasonography was performed to detect DVT. Similarly to the management described in the previous reports [10,11], all patients underwent anticoagulant therapy using unfractionated heparin immediately after being diagnosed with DVT. Because the anticoagulant therapy prior to surgery was effective in study patients, they were able to undergo systematic para-aortic and pelvic lymphadenectomy. The therapy was administered to these patients after surgery. We did not perform the systematic lymphadenectomy for the patients in whom the placement of inferior vena cava (IVC) filter before surgery was used for preventing lethal pulmonary thromboembolism with massive DVT.
Data are presented as frequencies and percentages for categorical factors and mean±standard deviation of interest for continuous variables. The associations between the degree of iliac vein compression and the clinical and laboratory variables (age, body mass index, primary site, clinical stage, number of lymph nodes removed, lymph node metastasis, D-dimer level) were statistically analyzed using the Kruskal-Wallis test. p-values <0.05 were considered statistically significant. In addition, in the patients with D-dimer levels >1.0 µg/mL, the associations between the presence of DVT and the clinical and laboratory variables including the factor of the degree of iliac vein compression were analyzed using the Spearman's rank test.
During the study period, 127 patients underwent systematic para-aortic and pelvic lymphadenectomy during surgery for primary gynecologic cancer at Chiba University Hospital. Eight patients were excluded because the surgical anatomy of the iliac artery and vein was not recorded. A total of 119 patients were included in this study and their characteristics and surgical data are summarized in Table 1.
Twenty-eight patients (23.5%) were in grade A with a calculated percentage of 0%-25% iliac vein compression, 47 patients (39.5%) were in grade B with a calculated percentage of 26%-50% compression, and 44 patients (37%) were in grade C with a calculated percentage of more than 50% compression. In most cases, the right common iliac artery compressed the left common iliac vein against the vertebrae. Compression of the left common iliac vein by both right and left common iliac arteries was identified in 2 patients (1.7%) with grade C compression. The mean diameter of the common iliac artery was 10.8±0.85 mm (range, 8.8 to 12.9 mm). The mean diameter of the common iliac vein immediately distal to the crossing site was 17.8±2.86 mm (range, 12.0 to 22.5 mm).
Seven patients (5.9%) had common iliac veins demonstrating anomalous anatomies (Table 2). The iliac veins of five patients (4.2%) were severely compressed; three of these (2.5%) had iliac veins divided into small caliber vessels (Fig. 2), whereas the other two (1.7%) showed a flattened structure (Fig. 3). Double IVC were detected in the remaining two patients (1.7%), and there was almost no compression of the common iliac veins in either of them. During surgery for the case presented in Fig. 2, we ruptured one of the small caliber vessels at the site immediately above the right common iliac artery between the aorta and the IVC. Proximal and distal to the site to where the injured vessel connected, IVC and both sides of the common iliac veins were clamped and the injured vessel was repaired with sutures. It took a great deal of time and a significant amount of blood loss, which was estimated at 1,785 mL for 30 minutes, to establish hemostasis.
The mean plasma D-dimer level was 3.2 µg/mL (range, 0.2 to 31.3 µg/mL). Of the 65 patients with elevated D-dimer levels >1.0 µg/mL, DVT was detected in 19 patients (29.2%): left leg involvement in 12 patients, right leg involvement in 3 patients, and involvement of both legs in 4 patients. Besides DVT, seven patients (10.8%) had pulmonary thromboembolism. The degree of iliac vein compression was not associated with any of the clinical and laboratory variables (Table 3). Statistical analyses revealed that the presence of DVT was associated with only one factor, D-dimer level (Table 4). An association between the presence of DVT and the degree of iliac vein compression could not be noted.
Iliac vein compression syndrome, also termed May-Thurner syndrome or Cockett syndrome, is the symptomatic compression of the left common iliac vein between the right common iliac artery and the fifth lumbar vertebra. This syndrome occurs predominantly in young to middle-aged women, and there is a left-sided predominance of iliofemoral DVT secondary to venous stasis [7,8]. In 1957, May and Thurner [7] described the development of "spurs" within the vein wall as a consequence of compression of the left iliac vein by the right iliac artery against the lumbar vertebra. They theorized that it was a combination of both mechanical compression and arterial pulsations by the right iliac artery that led to development of intimal hypertrophy of the iliac vein wall. Metastatic lymph nodes were detected throughout the para-aortic and pelvic regions including the area around the crossing site of the common iliac artery over the common iliac vein in patients with ovarian cancer [12]. In this study, we described the variability in outward appearance of the common iliac veins and evaluated the relationship between the degree of iliac vein compression and the presence of DVT using the data from surgeries for gynecologic cancer.
Cadaver studies published in the first half of the twentieth century have reported obstructive lesions of the iliac vein in approximately 20%-30% specimens [7,13,14]. Using 430 cadavers, May and Thurner [7] found that the right iliac artery compressed the iliac vein against the fifth lumbar vertebra in 22%. Kibbe et al. [9] reported that 24% of 50 asymptomatic patients had more than 50% compression of the common iliac vein, as measured using CT. They reported that their results correlated well with earlier autopsy studies. In this study, 37% patients with gynecologic cancer had more than 50% compression of the common iliac vein. Cockett and Thomas [8] reported on the prevalence of iliac vein compression in females. Our study included only females; therefore, our data shows a relatively high incidence of iliac vein compression in females. The obstructive lesion of the common iliac vein in 2.5% patients was identified as small caliber vessels. This type of obstructive lesion did not apply to the one described in the previous studies [7,13,14]. Anomalies such as small caliber vessels and flattened structures should be taken into account by surgeons performing presacral lymphadenectomy. One of our patients suffered considerable blood loss during presacral lymphadenectomy. Following this operation, we carefully considered the anatomy of the common iliac vein and could thus perform lymphadenectomy around the common iliac arteries with greater safety.
Elevated D-dimer levels were associated with the incidence of DVT and pulmonary thromboembolism in patients with ovarian and endometrial cancer. Satoh et al. [11] observed that elevated D-dimer levels were associated with the presence of venous thromboembolism before treatment for ovarian cancer (D-dimer <1.5 µg/mL, 0%; D-dimer ≥1.5 µg/mL, 39%). Satoh et al. [10] also observed that 17 of the 37 endometrial cancer patients with elevated D-dimer levels ≥1.5 µg/mL (45.9%) displayed DVT and 8 (21.6%) showed pulmonary thromboembolism. Plasma D-dimer levels were measured in all gynecological cancer patients before treatment, and 1.0 µg/mL was used as a cut-off value at our institution. In this study, 29% patients with elevated D-dimer levels >1.0 µg/mL were diagnosed with DVT. This result was consistent with the study results of Satoh et al. who reported on patients with ovarian cancer and endometrial cancer. The anatomic variant of the left common iliac vein is commonly found in the asymptomatic general population [7,8]. Although the incidence of this anatomic variant is relatively high and the incidence of DVT is relatively low in the general population, the presence of this anatomic variant alone may not represent an increased risk of DVT [9]. An association between the degree of iliac vein compression and the incidence of DVT was not identified in this study. In patients with gynecologic cancer, the presence of pelvic malignancy may put them at greater risk of developing DVT than iliac vein compression.
The variability in vascular anatomy of the common iliac veins during live surgery for gynecologic cancer was investigated in this study. Severe compression of the common iliac veins was frequently observed and anomalous anatomies of them were noted in a portion of the gynecologic cancer patients during systematic para-aortic and pelvic lymphadenectomy.
Figures and Tables
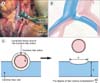 | Fig. 1(A) Intraoperative photograph of a patient during presacral lymphadenectomy. Upper side of the photograph is the cranial side of the patient. Length of the compressed common iliac vein (a) and the diameter of the common iliac artery (b) at the point where the common iliac artery crosses over the common iliac vein were measured. (B) Schematic representation of the surgical anatomy of the iliac veins and arteries in this patient. (C) Schematic representation of the calculation of the degree of iliac vein compression. CIA, common iliac artery. |
 | Fig. 2(A) Intraoperative photograph of a patient during presacral lymphadenectomy. Left side of the photograph is the cranial side of the patient. The left common iliac vein appeared to be small caliber vessels. One of them was ruptured and repaired with sutures (arrow heads). (B) Schematic representation of the surgical anatomy of the iliac veins and arteries in this patient. The ruptured vein is indicated by arrow heads. CIA, common iliac artery; CIV, common iliac vein; IVC, inferior vena cava. |
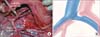 | Fig. 3(A) Intraoperative photograph of a patient during presacral lymphadenectomy. Left side of the photograph is the cranial side of the patient. In this case, the left common iliac vein was severely compressed and appeared to be a flattened structure (arrow heads). (B) Schematic representation of the surgical anatomy of the iliac veins and arteries in this patient. CIA, common iliac artery; CIV, common iliac vein. |
References
1. Chang SJ, Bristow RE, Ryu HS. Analysis of para-aortic lymphadenectomy up to the level of the renal vessels in apparent earlystage ovarian cancer. J Gynecol Oncol. 2013; 24:29–36.
2. Kim HS, Ju W, Jee BC, Kim YB, Park NH, Song YS, et al. Systematic lymphadenectomy for survival in epithelial ovarian cancer: a meta-analysis. Int J Gynecol Cancer. 2010; 20:520–528.
3. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–136.
4. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100:1707–1716.
5. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010; 375:1165–1172.
6. Wright JD, Herzog TJ, Abu-Rustum NR. Cytoreductive surgery: abdominal retroperitoneum and adenopathy. In : Bristow RE, Karlan BY, Chi DS, editors. Surgery for ovarian cancer. 2nd ed. New York: Informa Healthcare;2010. p. 122–139.
7. May R, Thurner J. The cause of the predominately sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957; 8:419–427.
8. Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg. 1965; 52:816–821.
9. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004; 39:937–943.
10. Satoh T, Matsumoto K, Uno K, Sakurai M, Okada S, Onuki M, et al. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br J Cancer. 2008; 99:1034–1039.
11. Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007; 97:1053–1057.
12. Takeshima N, Hirai Y, Umayahara K, Fujiwara K, Takizawa K, Hasumi K. Lymph node metastasis in ovarian cancer: difference between serous and non-serous primary tumors. Gynecol Oncol. 2005; 99:427–431.
13. McMurrich JP. The occurrence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1908; 135:342–346.
14. Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J. 1943; 26:737–750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download