Abstract
Objective
The objective of this study was to evaluate the effect of overexpression of epidermal growth factor receptor (EGFR) on the expression of epithelial cell markers (E-cadherin and α-catenin) and mesenchymal cell markers (N-cadherin and vimentin) in endometrial carcinoma.
Methods
The expression of all 4 markers was evaluated in EGFR overexpressing Ishikawa cells, control Ishikawa cells, and KLE cells using reverse transcription polymerase chain reaction (RT-PCR) and Western blotting. The expression of these 4 markers was also determined in cancerous tissues of patients with endometrial carcinoma using immunohistochemical staining.
Results
Ishikawa cells transfected with EGFR showed decreased expression of E-cadherin and α-catenin and increased expression of N-cadherin and vimentin compared with control Ishikawa cells (p<0.01 for all). The expression of N-cadherin and vimentin was higher and the expression of E-cadherin and α-catenin was lower in stage II-III than stage I and in grade II-III than grade I endometrial carcinoma tissue (p<0.01 for all).
Endometrial carcinoma is one of the most common gynecologic malignancies worldwide and is among the most invasive tumors of the female genital tract [1]. Localized recurrence and distant metastases are the main factors that negatively impact the prognosis of endometrial carcinoma patients [2]. Chemotherapy is the standard care for patients with advanced or recurrent disease, and progesterone-based hormone treatment is used for patients with early-stage endometrial carcinoma in which there is evidence for expression of the progesterone receptor (PR) [3].
During normal embryonic development, the epithelial-mesenchymal transition (EMT) is a process in which epithelial cells lose their polarity and cell adhesion properties and acquire a migratory phenotype [4]. EMT also plays a major role in tumor invasion and distant metastasis [5], and activation of EMT is associated with increased motility and invasiveness of tumor cells [6]. Numerous transcription factors (Snail, Twist, and Zeb) [7,8] and signaling pathways (TGF-β, NFκB, Wnt, and Notch) [9,10] regulate EMT. Downregulation of epithelial markers (E-cadherin and α-catenin) and upregulation of mesenchymal markers (N-cadherin and vimentin) are used to follow the progression of EMT [11,12]. A previous report indicated that expression of E-cadherin, α-catenin, and β-catenin were independent and positive prognostic factors for survival in endometrial carcinoma patients [13]. Another report indicated that EMT was associated with histologic grade and stage, myometrial invasion, and prognosis in patients with endometrial carcinoma [1].
Epidermal growth factor receptor (EGFR) family members, including EGFR (Her1), ERBB2 (Her2/neu), ERBB3, and ERBB4, are highly expressed in endometrial carcinomas and have been the focus of targeted therapies of endometrial carcinoma [14]. Previous research indicated that overexpression of EGFR correlated with drug resistance, and cancer progression presumably due to inappropriate activation of EGFR signaling pathways [15,16]. However, there is limited information on the mechanism of EGFR in promoting the invasion and metastasis of endometrial carcinoma, and it is not clear if EGFR is associated with EMT.
We previously showed that EGFR was overexpressed in a highly invasive, poorly differentiated endometrial carcinoma cell lines (KLE cells) relative to a less invasive, well differentiated endometrial carcinoma cell lines (Ishikawa cells) [16]. We also showed that EGFR overexpression correlated with inappropriate activation of the EGFR/MAPK signaling pathway, reduced the expression of PR-B, and increased the progestin resistance of endometrial carcinoma cells [16]. The difference in EGFR expression of the 2 cell lines makes them ideal for determination of the relationship between EGFR and EMT in endometrial carcinoma.
In this study, we analyzed the expression of EGFR and different markers such as E-cadherin, α-catenin, N-cadherin, and vimentin in cancerous tissues of patients with endometrial carcinoma. EGFR overexpressing Ishikawa cells, control Ishikawa cells and KLE cells were used to investigate the correlation between overexpression of EGFR and mesenchymal markers.
Human endometrial carcinoma Ishikawa cells and KLE cells were obtained from the Sixth People's Hospital, Shanghai Jiao Tong University, China. The cells were cultured in Dulbecco Modified Eagle Medium-F12 Medium (Hyclone Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine serum, 100 µg/mL streptomycin, 100 U/mL penicillin, and 2 mM/L glutamine (Hyclone) and maintained at 37℃ in a humidified atmosphere with 5% carbon dioxide. The EGFR-Ishikawa cell line was established by transfection of Ishikawa cells with EGFR plasmids and selection of stable transfectants in G418-containing medium (600 mg/L; Invitrogen, Carlsbad, CA, USA) as previously described [16]. EGFR over-expression was verified by polymerase chain reaction (PCR) and western blotting.
Endometrial tissue samples were obtained from endometrial carcinoma patients who presented at the Sixth People's Hospital of Shanghai Jiao Tong University between 2005 and 2009. These patients were also examined in our previous study of the overexpression of EGFR in endometrial carcinoma [16]. Surgical stage and tumor grade was determined based on criteria of the International Federation of Gynecology and Obstetrics (FIGO) staging system. There were 40, 14, and 8 patients with grade 1, 2, and 3 tumors, respectively. Twenty-seven, twenty-four, and eleven patients had stage I, II, and III disease, respectively. The mean patient age was 55.5 years (range, 21 to 76 years). The study was performed in accordance with human subject guidelines and was approved by the Scientific and Ethical Committee of Shanghai Jiao Tong University. All subjects gave written informed consent.
Total RNA was extracted from endometrial carcinoma cells using Takara RNAiso Plus (Takara Shuzo, Kyoto, Japan) according to the manufacturer's directions. Total RNA (1 µg) was reverse transcribed into cDNA using a reverse transcription kit (Takara Shuzo). Real-time quantitative reverse transcription PCR (RT-PCR) was performed using 2 µL cDNA with the Light-Cycler 3.0 Real-Time PCR Detection System (Roche Diagnostics, Indianapolis, IL, USA) and the SYBR Green I PCR Kit (Takara Shuzo) according to the manufacturers' instructions. PCR amplification was performed under the following conditions: 95℃ for 10 seconds, 40 cycles at 95℃ for 5 seconds, 62℃ for 30 seconds, and 65℃ for 15 seconds.
The PCR primers for the EGFR, E-cadherin, α-catenin, N-cadherin, and vimentin genes were designed and synthesized by Takara. The primers for EGFR were 5'- GACAGCTATGAGATGGAGGAA -3'(sense) and 5'- GAGTCACCCCTAAATGCCA -3' (antisense); the primers for E-cadherin were 5'- TACACTGCCCAGGAGCCAGA -3'(sense) and 5'- TGGCACCAGTGTCCGGATTA -3' (antisense); the primers for α-catenin were 5'- CTCTACTGCCACCAGCTGAACATC -3'(sense) and 5'- ATGCCTTCACTGTCTGCACCAC -3' (antisense); the primers for N-cadherin were 5'- CGAATGGATGAAAGACCCATCC -3'(sense) and 5'- TAGCAGCTTCAACGGCAAAGTTC -3' (antisense); the primers for vimentin were 5'- TGAGTACCGGAGACAGGTGCAG -3'(sense) and 5'- GGAGCCACTGCCTTCATAGTCAA -3' (antisense). GAPDH was used as the reference gene, and primers for GAPDH were 5'- GCACCGTCAAGGCTGAGAAC -3' (sense) and 5'- ATGGTGGTGAAGACGCCAGT -3' (antisense). PCR products were evaluated by melting curve analysis.
Cells were grown to 70%-80% subconfluence and harvested in a RIPA lysis buffer containing 0.15M NaCl, 1% NP40, 0.01M deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.05 M Tris-HCl, pH 8.0, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 10 µg/mL each of aprotinin, pepstatin, and leupeptin. Equal amounts of protein were electrophoresed on SDS-polyacrylamide gels (10% for vimentin and GAPDH; 7.5% for EGFR, E-cadherin, α-catenin, and N-cadherin). Protein was transferred onto polyvinylidene difluoride (PVDF) membranes and blocked in 5% nonfat dry milk, 0.1% Tween-20, and PBS for 1 hour at room temperature. The membranes were incubated overnight at 4℃ with a 1:10,000 dilution of rabbit anti-EGFR monoclonal antibody, rabbit anti-E-cadherin monoclonal antibody, rabbit anti-α-catenin monoclonal antibody, rabbit anti-N-cadherin monoclonal antibody, or a 1:5,000 dilution of rabbit anti-vimentin monoclonal antibody (all primary antibodies from Epitomics Inc., Burlingame, CA, USA). A 1:5,000 dilution of mouse anti-GAPDH monoclonal antibody (KangChen BIo-tech, Shanghai, China) was used as a normalizing control. The blots were washed in 0.1% Tween-20 with PBS and then incubated with secondary antibodies at room temperature for 1 hour. The results were analyzed by use of enhanced chemiluminescence (Pierce, Rockford, IL, USA), photographed by an electrophoresis imaging system, and scanned and quantified with Image J software.
Expression of E-cadherin, α-catenin, N-cadherin, and vimentin proteins was evaluated in endometrial carcinoma tissue by immunohistochemistry using the avidin-biotin complex/immunoperoxidase method. Briefly, deparaffinized sections were treated with 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were then blocked with 5% normal serum for 20 minutes and incubated with a 1:100 dilution of rabbit anti-E-cadherin, α-catenin, N-cadherin, and vimentin monoclonal antibody overnight at 4℃. The sections were rinsed with PBS, incubated with biotinylated secondary antibodies and avidin-biotin complex/horseradish peroxidase and visualized with 3, 3'-diaminobenzidine. The sections were counterstained with hematoxylin. The slides were independently evaluated by 2 investigators who had no knowledge of the clinical or pathologic parameters of the patients. In addition, the slides were also evaluated by a double-blind method by two professors of the Department of Pathology of Shanghai Jiaotong University Affiliated Sixth People's Hospital.
Samples were viewed at high magnification (×400) and the total score was based on a system previously described by Fromowitz et al. [17] with a minor modification that considered the specific expression pattern and localization of each marker. Expression of each marker was determined by randomly selecting 10 fields and counting the number of positive cells among 1,000 cells. Expression was scored as the sum of staining intensity and the number of positive cells. The number of positive cells was scored as: 0 for no positive cells, 1 for 1%-10% positive, 2 for 11%-50% positive, and 3 for more than 50% positive. Staining intensity was scored as follows: 0 for no staining, 1 for light yellow, 2 for yellow-brown, and 3 for brown. Final scores were 0 for negative, 1 for positive (+), 2 to 3 for moderately positive (++), and more than 3 for strongly positive (+++).
The results of the RT-PCR, western blotting, and immunohistochemistry experiments (continuous variables) are presented as means and standard deviations and compared using ANOVA with a Bonferroni correction or using the independent t-test. All statistical analyses were performed with SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant.
We examined the histology of Ishikawa cells to confirm that they had an epithelial phenotype with tight junctions and lateral, apical and base membranes. The results indicate that Ishikawa cells were circular, elliptical, or polygonal in shape (Fig. 1A, B). However, Ishikawa cells that overexpressed EGFR (EGFR-Ishikawa) had a mesenchymal phenotype, with no close intra-cellular interactions (Fig. 1C, D). Most of these EGFR-Ishikawa cells were spindle shaped and the cell membranes were extended into pseudopods, related to their enhanced motility. EGFR-Ishikawa cells also had increased intercellular spaces compared to control Ishikawa cells, suggesting a decline in cell adhesion. EGFR-Ishikawa cells were non-polarized and produced a definite extracellular matrix.
We used RT-PCR to measure the mRNA levels of EGFR, epithelial markers (E-cadherin and α-catenin) and mesenchymal markers (N-cadherin and vimentin) in KLE cells, EGFR-Ishikawa cells, and control Ishikawa cells (Fig. 2). Single factor analysis of variance indicated that KLE cells and EGFR-Ishikawa cells had statistically significant downregulation of α-catenin and upregulation of N-cadherin and vimentin. The downregulation of E-cadherin in KLE cells was not statistically significant. EGFR-Ishikawa cells had a significant downregulation of E-cadherin and α-catenin and upregulation of N-cadherin and vimentin relative to the control Ishikawa cells (p<0.01 for all). Further analysis showed a negative correlation between the expression of EGFR and epithelial markers, and a positive correlation between the expression of EGFR and mesenchymal markers.
Next, we used western blotting to measure the expression of EGFR and the same 4 markers in KLE cells, EGFR-Ishikawa cells, and control Ishikawa cells (Fig. 3). The immunoblotting data are consistent with the RT-PCR data. In particular, these data show that E-cadherin was significantly downregulated and N-cadherin and vimentin were significantly upregulated in KLE cells relative to Ishikawa cells (p<0.01 for all). E-cadherin and α-catenin were significantly downregulated and N-cadherin and vimentin significantly upregulated in EGFR-Ishikawa cells relative to control Ishikawa cells (p<0.01 for all). Further analysis showed a negative correlation between expression of EGFR and epithelial markers, and a positive correlation between expression of EGFR and mesenchymal markers.
Finally, we used immunohistochemistry followed by image analysis for quantitation to determine the expression of epithelial and mesenchymal markers in the endometrial tissues of patients with endometrial carcinoma (Table 1, Fig. 4). First, we grouped study participants based on age: younger than 50 years; 50-64 years; older than 64 years. The results indicate significantly higher expression of E-cadherin in the middle age group relative to the other 2 age groups (Table 1). Single factor analysis of variance indicated no statistically significant differences in the expression of α-catenin, N-cadherin, and vimentin in the 3 age groups (p>0.05). In addition, multiple regression analysis indicated no significant linear relationship between the expression of E-cadherin, α-catenin, N-cadherin, and vimentin and patient age (t values: -0.70, -0.54, 0.50, 0.61, respectively; p>0.05). We also classified these endometrial carcinoma tissues based on FIGO stage (stage I vs. stages II, III) and grade (G1 vs. G2, 3). The results indicated that expression of E-cadherin and α-catenin were significantly higher in tissues classified as stage I and as grade I, and that expression of N-cadherin and vimentin were significantly higher in tissues classified as stage II-III and as grade II-III (Table 1, Fig. 4).
In this study, KLE cells had significantly higher levels of EGFR and of 2 mesenchymal cell markers (N-cadherin and vimentin) and significantly lower levels of 2 epithelial cell markers (E-cadherin and α-catenin) compared to Ishikawa cells. Ishikawa cells that exhibited stable overexpression of EGFR had decreased expression of E-cadherin and α-catenin and increased expression of N-cadherin and vimentin. In addition, expression of mesenchymal cell markers was significantly higher and expression of epithelial cell markers was significantly lower in the tumors of patients with late stage and high grade endometrial carcinoma. Although the differences in expression that we observed were statistically significant, some of the differences were rather small (e.g., only ~2-fold change for EGFR), suggesting that other factors may play more important roles in the pathogenesis of endometrial carcinoma. We observed no correlation between patient age and markers of EMT.
Numerous transcription factors and signaling pathways play roles in regulating EMT during embryonic development [18]. For example, TGFβ-R signaling and an activated MAPK pathway are required for EMT, and for cell migration and invasion [19]. Several signaling pathways, including the EGFR, VEGFR and PI3K/PTEN/AKT/mTOR pathways, are currently being investigated as therapeutic targets for endometrial carcinoma [20]. Numerous molecular signals are involved in the induction of EMT, including (1) activation of Rho small GTPases (Rac1/RhoA), which increase cell motility [21]; (2) nuclear translocation of transcription factors such as Snail and Twist [18,22]; and (3) dysregulation of the EGFR/MAPK signaling pathway [23]. Previous research suggested that EGFR activates Ras, which regulates Rac and Rho via PI3K. Rac and Rho are thought to play a role in EMT by regulation of adherens junctions, focal adhesions, myosin phosphorylation, actin stress fibers, motility, and scattering. Ras-Raf signaling also activates the MAPK cascade and subsequently, Snail, which regulates E-cadherin transcription by binding to the E-box of the E-cadherin gene [23]. E-cadherin is known to act as a tumor suppressor and its expression correlates with cell invasion [24] and histologic grade in endometrial carcinoma [25]. Sakuragi et al. [25] reported decreased E-cadherin expression was associated with unfavorable clinical tumor characteristics, such as dedifferentiation and deep myometrial invasion. Hipp et al. [26] demonstrated that the transcription factor Snail, one of the most prominent transcriptional E-cadherin repressors, was regulated by activation of EGFR. In EGFR-Ishikawa cells, EGF leads to activation of the AKT and ERK1/2 pathways, which results in upregulation of the Snail protein, but not of Snail mRNA [26]. In the present study, we found significant downregulation of E-cadherin mRNA, but only modest downregulation of E-cadherin protein in EGFR-Ishikawa cells relative to KLE cells and Ishikawa cells. The aforementioned AKT/mTOR pathways are considered as potential therapeutic targets for endometrial carcinoma [20], but our results provide indirect evidence of the complexity of EGFR signaling pathways in Ishikawa cells, EGFR-Ishikawa cells, KLE cells, and endometrial carcinoma tissues.
There are several limitations of this study. We did not perform functional assays to evaluate invasion or cell migration in the context of EGFR levels and EMT. Second, we did not have data on the regulation of transcription factors and signaling pathways, such as Snail. Finally, we did not have clinical outcome data and were unable to perform survival analysis of the endometrial carcinoma patients.
In conclusion, our results indicated that decreased expression of epithelial markers (E-cadherin and α-catenin) and increased expression of mesenchymal markers (N-cadherin and vimentin) were observed in human endometrial carcinoma tissue as well as together with high EGFR expression correlation in cultured endometrial carcinoma cells.
Figures and Tables
Fig. 1
H&E staining of endometrial carcinoma cells. (A) Control Ishikawa cells (×200). (B) Control Ishikawa cells (×400). (C) Epidermal growth factor receptor (EGFR)-Ishikawa cells (×200). (D) EGFR-Ishikawa cells (×400).

Fig. 2
Expression of epidermal growth factor receptor (EGFR), E-cadherin, α-catenin, N-cadherin, and vimentin mRNAs in Ishikawa cells, KLE cells, and EGFR-Ishikawa cells. Expression was determined by reverse transcription polymerase chain reaction. *p<0.01 vs. Ishikawa cells.

Fig. 3
Expression of epidermal growth factor receptor (EGFR), E-cadherin, α-catenin, N-cadherin, and vimentin proteins in Ishikawa cells, KLE cells, and EGFR-Ishikawa cells. A representative western blot is shown (above) and image analysis of 3 separate experiments was used for quantitation (below). *p<0.01 vs. Ishikawa cells.
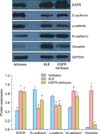
Fig. 4
Immunohistochemical staining of epithelial and mesenchymal markers in endometrial carcinoma tissues (×400). A-D are tissues from one representative patient (stage I, grade 1), and panels E-H are tissues from another representative patient (stage II, grade 3). The staining was for E-cadherin (A and E); α-catenin (B and F); N-cadherin (C and G); and vimentin (D and H).

References
1. Abal M, Llaurado M, Doll A, Monge M, Colas E, Gonzalez M, et al. Molecular determinants of invasion in endometrial cancer. Clin Transl Oncol. 2007; 9:272–277.
2. Tsukamoto H, Shibata K, Kajiyama H, Terauchi M, Nawa A, Kikkawa F. Irradiation-induced epithelial-mesenchymal transition (EMT) related to invasive potential in endometrial carcinoma cells. Gynecol Oncol. 2007; 107:500–504.
3. Yang S, Thiel KW, De Geest K, Leslie KK. Endometrial cancer: reviving progesterone therapy in the molecular age. Discov Med. 2011; 12:205–212.
4. Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004; 118:277–279.
5. Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008; 100:232–239.
6. Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000; 60:1091–1099.
7. Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010; 21:Suppl 7. vii89–vii92.
8. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010; 29:4741–4751.
9. Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci. 2010; 67:2605–2618.
10. Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011; 1:29.
11. Saitoh M, Shirakihara T, Miyazono K. Regulation of the stability of cell surface E-cadherin by the proteasome. Biochem Biophys Res Commun. 2009; 381:560–565.
12. Cavallaro U, Schaffhauser B, Christofori G. Cadherins and the tumour progression: is it all in a switch? Cancer Lett. 2002; 176:123–128.
13. Scholten AN, Aliredjo R, Creutzberg CL, Smit VT. Combined E-cadherin, alpha-catenin, and beta-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer. 2006; 16:1379–1385.
14. Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009; 16:8–13.
15. Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004; 11:623–641.
16. Ai Z, Wang J, Wang Y, Lu L, Tong J, Teng Y. Overexpressed epidermal growth factor receptor (EGFR)-induced progestin insensitivity in human endometrial carcinoma cells by the EGFR/mitogen-activated protein kinase signaling pathway. Cancer. 2010; 116:3603–3613.
17. Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, et al. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987; 18:1268–1275.
18. Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000; 2:76–83.
19. Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998; 8:1243–1252.
20. Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011; 8:261–271.
21. Boivin D, Bilodeau D, Beliveau R. Regulation of cytoskeletal functions by Rho small GTP-binding proteins in normal and cancer cells. Can J Physiol Pharmacol. 1996; 74:801–810.
22. Teng Y, Zeisberg M, Kalluri R. Transcriptional regulation of epithelial-mesenchymal transition. J Clin Invest. 2007; 117:304–306.
23. Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, et al. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007; 117:482–491.
24. Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991; 113:173–185.
25. Sakuragi N, Nishiya M, Ikeda K, Ohkouch T, Furth EE, Hareyama H, et al. Decreased E-cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol. 1994; 53:183–189.
26. Hipp S, Walch A, Schuster T, Losko S, Laux H, Bolton T, et al. Activation of epidermal growth factor receptor results in snail protein but not mRNA overexpression in endometrial cancer. J Cell Mol Med. 2009; 13:3858–3867.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download