Abstract
Objective
The purpose of this study was to evaluate the expression of epidermal growth factor-like domain 7 (EGFL7) in epithelial ovarian cancer, and to assess its relevance to clinicopathological characteristics and patients' survival.
Methods
A total of 177 patients with epithelial ovarian cancer were enrolled in the current study. For each patient, a retrospective review of medical records was conducted. Immunohistochemical staining for EGFL7 was performed using tissue microarrays made with paraffin-embedded tissue block. EGFL7 expression levels were graded on a grade of 0 to 3 based on the percentage of positive cancer cells. We analyzed the correlations between the expression of EGFL7 and various clinical parameters, and also analyzed the survival outcome according to the EGFL7 expression.
Results
The expression of EGFL7 in ovarian cancer tissues was observed in 98 patients (55.4%). High expression of EGFL7 (grade 2 or 3) was significantly correlated with pathologic type, differentiation, stage, residual tumor after debulking surgery, lymphovascular space involvement, lymph node metastasis, high cancer antigen 125, peritoneal cytology, and ascites. Among these clinicopathologic factors, differentiation was significantly correlated with EGFL7 expression in multivariate analysis (p<0.05). Survival analysis showed that the patients with high EGFL7 expression had a poorer disease free survival than those with low EGFL7 expression (p=0.002).
Epithelial ovarian cancer is still the most lethal of all gynecologic malignancies, and it is the fourth most common cause of cancer-related death among women worldwide [1]. Because proper screening test for early detection is not available, most of the ovarian cancer patients are diagnosed in the advanced stage, which leads to a high recurrence rate and poor prognosis. Therefore, new therapeutic strategies and new methods for early detection are needed.
Epidermal growth factor-like domain 7 (EGFL7) also known as vascular endothelial-statin (VE-statin) is a protein encoded by the EGFL7 gene. The EGFL7 gene is mostly expressed in endothelial cells. Expression of EGFL7 is endothelial cell-specific in physiologic conditions, such as in, reproductive organs during pregnancy and vascular injury. However, EGFL7 is aberrantly expressed by tumor cells in human cancers, including colorectal cancer, hepatocellular carcinoma, breast cancer, laryngeal carcinoma, and malignant glioma [2,3,4,5,6]. Studies showed that the overexpression of EGFL7 is correlated with advanced stage, lymph node metastasis, vascular invasion, metastasis, tumor grade, and poor prognosis [2,3,4,5,7]. Association of EGFL7 with mechanism of immune evasion has also been reported. The study showed that EGFL7 expression in tumors promotes tumor progression by reducing the expression of endothelial adhesion molecules, which results in fewer immune cell infiltrations [8].
To date, only a limited studies have reported EGFL7 expression in ovarian cancer [5,9], and the studies reported EGFL7 expression status in ovarian cancer cell lines or in a limited number of patients. Furthermore, none of the studies showed the relationship between the EGFL7 expression and prognosis in ovarian cancer. The purpose of this study was to evaluate the expression of EGFL7 in epithelial ovarian carcinoma, and to assess its relevance to clinicopathological characteristics and survival outcome.
We searched for patients with epithelial ovarian cancer who underwent staging operations with or without adjuvant chemotherapy from an oncology database maintained at the Department of Obstetrics and Gynecology in Daegu Catholic University. Inclusion criteria were as follows: (1) patients with epithelial ovarian cancer (query term: serous, mucinous, clear, endometrioid, transitional, malignant Brenner); (2) patients who underwent primary therapy in the same institution; and (3) patients diagnosed between January 2000 and September 2011. We excluded the patients who received neoadjuvant chemotherapy before primary debulking surgery. This retrospective study was approved by the Institutional Review Board of Daegu Catholic University Medical Center.
Applying the inclusion criteria, a total of 177 patients with epithelial ovarian cancer were enrolled in the current study. For each patient, a retrospective review of medical records was conducted. The histologic slides of the surgically removed ovarian cancer tissues were reviewed again by two independent pathologists, who were blinded to the clinical variables of patients.
Overall survival (OS) was defined as the time from the diagnosis (the date of surgery) to death from epithelial ovarian cancer. Patients who survived beyond the time of analysis were censored at the time of their last follow-up date. Disease-free survival (DFS) was defined as the time from the diagnosis to the recurrence of cancer in any of the sites.
Representative paraffin tumor blocks were selected according to the primary evaluation of H&E stained slides of ovarian cancer before they were prepared for tissue microarrays (TMA). Two tumor tissue cores (1 mm in diameter) were taken from each of the donor ovarian cancer tissue blocks with a manual punch arrayer (Quick-Ray, Uni-Tech Science, Seoul, Korea). The cores were placed in a new recipient paraffin block that ultimately contained 59 to 91 tissue cores. Multiple sections (5 µm in thickness) were cut from the TMA blocks and then mounted onto microscope slides. The TMA H&E stained sections were reviewed under light microscopy to confirm the presence of representative tumor areas.
Immunohistochemistry was conducted on 5 µm thick TMA tissue sections using the Bond Polymer Intense Detection System (Leica Microsystems, Mount Waverley, Australia) according to the manufacturer's instructions with minor modifications. Briefly, the 5-µm thick sections of formalin-fixed and paraffin-embedded TMA tissues were deparaffinized with Bond Dewax Solution (Leica Microsystems), and an antigen retrieval procedure was performed using Bond ER Solution (Leica Microsystems) for 30 minutes at 100℃. The endogenous peroxidase activity was quenched following 5-minute incubation with hydrogen peroxide. Sections were incubated for 15 minutes at ambient temperature with a mouse monoclonal anti-EGFL7 antibody (1:100; Abcam, Cambridge, MA, USA) using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system in a Bond-Max automatic slide stainer (Leica Microsystems). Negative control slides were probed with normal mouse serum under the same experimental conditions.
EGFL7 expression levels in ovarian cancer tissue were graded on a scale of 0 to 3 based on the cytoplasmic and membrane staining intensity of positive tumor cells by two independent pathologists who were blinded to the patients' clinical records. The staining was graded to using a 4-point scale according to the percentage of positive cancer cells: grade 0 (≤10% positive); grade 1 (11% to 25% positive); grade 2 (26% to 50% positive); grade 3 (≥51% positive) (Fig. 1) [4,5]. Immunohistochemical staining for EGFL7 in ovarian cancer tissue was also classified as low (grade 0 or 1) or high (grade 2 or 3) [5].
Comparisons of variables between the groups were based on the chi-square test and independent samples t-test. The OS and DFS were estimated by the life-table method of Kaplan-Meier. Differences in survival rates were assessed by the log-rank test. Multivariate analysis was performed by logistic regression analysis and Cox regression hazard model. The p-values were the result of two-sided tests, and p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS ver. 19 (IBM Co., Armonk, NY, USA).
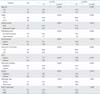
Characteristics of enrolled patients, and the relationship between EGFL7 expression and clinicopathologic characteristics of epithelial ovarian cancer are shown in Table 1. In terms of histologic types, serous adenocarcinoma accounted for 40.7%, mucinous adenocarcinoma for 24.9%, endometrioid adenocarcinoma for 18.1%, clear cell carcinoma for 13.6%, and transitional cell carcinoma for 1.7%, and others for 1.1%, respectively. The number of patients in each FIGO stage was as follows: stage IA 46, IB 3, IC 19, IIA 2, IIB 7, IIC 11, IIIA 2, IIIB 10, IIIC 64, and IV 13 (Table 1). Median follow-up time was 44.0 months, and the 5- and 10-year survival rate in whole series was 59.2% and 56.6%, respectively.
Our study showed that EGFL7 protein mainly expressed in the cytoplasm of ovarian cancer cells, although weak EGFL7 expression could also be found in endothelial cells (Fig. 1). This finding was similar to that of the previous study [5]. The positive expression of EGFL7 (grade 1 to 3) in ovarian cancer tissues was observed in 98 patients (55.4%). Survival analysis showed that there was no difference in survival between patients with grade 0 EGFL7 expression and those with grade 1 EGFL7 expression (5-year OS of patients with grade 0 and grade 1 EGFL7 expression; 64.8% and 62.3%, respectively, p>0.05). However, patients with grade 2 or 3 EGFL7 expression showed poorer survival than those with grade 0 or 1 EGFL7 expression. Based on the results, we grouped grade 0 and grade 1 EGFL7 expression as 'low EGFL7 expression', and grade 2 and grade 3 EGFL7 expression as high EGFL7 expression' for further analysis.
High EGFL7 expression was associated with the pathologic type, differentiation, stage, gross residual tumor after debulking surgery, lymph node metastasis, high serum cancer antigen 125 (CA-125), lymphovascular space involvement (LVSI), peritoneal cytology, and presence of ascites (Table 1). Among these clinicopathologic parameters, multivariate analysis by logistic regression showed that EGFL7 expression was significantly correlated with differentiation (odds ratio, 0.074; 95% confidence interval, 0.016 to 0.356; p=0.001), but not with other parameters.
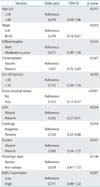
Survival analysis showed that age, stage, differentiation, optimal debulking, lymph node metastasis, high serum CA-125, LVSI, peritoneal cytology, ascites, and pathologic type were significantly correlated with overall and disease free survival (Table 2). Survival analysis showed that the patients with high EGFL7 expression had a poorer DFS than those with low EGFL7 expression (p=0.002) (Fig. 2). A similar tendency for difference in survival was observed in OS, but the difference did not reach statistical significance (p=0.142) (Table 2). However, Cox multivariate regression analysis showed that EGFL7 expression was not an independent prognostic factor (Table 3).
EGFL7 gene, also known as NEU1, ZNEU1, VE-STATIN, RP11-251M1.2, is a novel gene that was first identified in endothelial cells of the developing mouse embryo, and the human EGFL7 gene is located on the long arm of chromosome 9 [10,11]. EGFL7 is a secreted protein that is encoded by the EGFL7 gene, which was initially described as a 30 kD protein exclusively expressed by VE cells [12]. EGFL7 is highly expressed during embryonic development, but upon birth, it becomes down-regulated in the vascular system. In the adult vasculature, expression of EGFL7 is restricted to sites of active vascular remodeling, and it acts on endothelial cells to control blood vessel development during both physiologic and pathologic angiogenesis, e.g., during menstruation, wound healing, tumor growth, or metastasis [13].
The EGFL7 expression of human normal ovarian tissue has not been known precisely. Fan et al. [5] reported the expression of EGFL7 in 19 kinds of adult human normal tissues, but they did not evaluate that in human normal ovarian tissue. An animal study showed that EGFL7 is expressed by primordial germ cells during mouse embryonic development, but after differentiation, the EGFL7 expression in the ovary is down-regulated and remains only limited in the vascular endothelium [14]. In the current study, we evaluated the EGFL7 expression of normal ovarian tissues from seven patients who underwent other surgery, and none of the normal ovarian tissues of the patients showed positive expression of EGFL7.
Recently, increased EGFL7 expression was also reported in human colon cancer, breast cancer, hepatocellular carcinoma, and laryngeal squamous cell carcinoma, and it was closely correlated with lymph node metastasis, advanced tumor stage, and poor prognosis [2,3,5,6]. In the current study, similar findings were observed; EGFL7 expression was found in epithelial ovarian cancer cells, and it was significantly correlated with tumor grade and poor prognosis. The role of EGFL7 in tumor angiogenesis, cell proliferation, and mechanism of immune evasion has also been reported [4,5,8,10,13]. However, evidence for the function of EGFL7 in human malignancies is still limited [6,15].
With regard to the relationship between EGFL7 expression and survival outcome in various cancers, most of studies showed that increased EGFL7 expression is correlated with poor prognosis and many poor clinicopathologic prognostic factors including advanced tumor stage, lymph node metastasis, and tumor grade [2,3,4,5,6]. However, a contrary result has also been reported in one study, which showed that the expression of EGFL7 was correlated with low-grade invasive lesion, absence of axillary lymph node metastasis, and good prognosis in human breast cancer [15]. In the current study, we observed that high EGFL7 expression was correlated with various kinds of poor clinicopathologic prognostic factors including poor differentiation, advanced stage, lymph node metastasis, high serum CA-125, LVSI, positive peritoneal cytology, grossly residual tumors, and ascites, which lead to poor DFS. In particular, tumor differentiation was significantly related with high EGFL7 expression in multivariate analysis. A similar finding was reported in another study, in which EGFL7 expression in malignant glioma was significantly correlated with tumor grade [3].
To date, the expression of EGFL7 in ovarian cancer has been evaluated only in a few studies [5,9], and none of these studies investigated the relationship between the expression of EGFL7 and survival outcome in ovarian cancer. Fan et al. [5] evaluated the expression of EGFL7 in normal adult human tissues and 10 kinds of human epithelial cancer tissues including ovarian cancer. With respect to ovarian cancer, the authors evaluated the expression of EGFL7 in fresh ovarian cancer tissue obtained from three patients, and in paraffin-embedded specimens obtained from six patients. The study investigated the correlation between the expression of EGFL7 and survival outcome in hepatocellular carcinoma and breast cancer, but not in ovarian cancer. They found that EGFL7 was differentially expressed in 19 adult human normal tissues and was overexpressed in all 10 human epithelial tumor tissues, and increased EGFL7 expression in hepatocellular carcinoma and breast cancer was correlated with poor prognosis. Fan and colleagues [5] also found that the serum EGFL7 level was also significantly elevated in cancer patients. Another study reported that NEU1 (synonym for EGFL7) and NEU3 expression levels were found to be elevated in most of the cell lines among the 18 cell lines derived from human ovarian cancers, but the study focused on NEU3 expression in clear cell ovarian carcinoma [9].
In this study, a significantly poorer DFS was observed in patients with high EGFL7 expression than in those with low EGFL7 expression, but the difference in OS did not reach statistical significance. This might have been caused by limited number of enrolled subjects (the number of patients showing high EGFL7 expression was only 54). Another explanation could be that not all of the recurred patients died because some of the patients with recurrent disease were salvaged by secondary treatment, e.g., secondary debulking surgery with adjuvant chemotherapy.
Recently, a preclinical trial raised a possibility of cancer antiangiogenic treatment using anti-EGFL7 antibodies [10,16]. Johnson et al. [16] demonstrated that administration of anti-EGFL7 antibodies enhanced both stress-induced endothelial cell death and therapeutic effect of antivascular endothelial growth factor (anti-VEGF) therapy in non-small cell lung cancer xenograft model. Currently, clinical trials are being conducted to evaluate the effect of combined anti-EGFL7 and Avastin (anti-VEGF Ab) therapy on tumor vascular function and growth [10]. It is expected that anti-EGFL7 therapy in conjunction with anti-VEGF therapy will provide additional inhibition of angiogenesis in various kinds of cancers.
In conclusion, we found that high EGFL7 expression in epithelial ovarian cancer was correlated with poor differentiation and other poor prognostic factors, which led to poor DFS. This result is in line with the results observed in other types of cancer [2,3,4,5,6]. Our data suggest that EGFL7 expression is a novel predictive factor for the clinical progression of epithelial ovarian cancer, and EGFL7 may constitute a therapeutic target for antiangiogenesis therapy in patients with epithelial ovarian cancer, i.e., anti-EGFL7 therapy can be applied to the treatment of epithelial ovarian cancer.
Figures and Tables
 | Fig. 1Representatives of epidermal growth factor-like domain 7 expression of each grade in immunohistochemical staining (×100). The staining was graded to using a 4-point scale according to the percentage of positive cancer cells: (A) grade 0 (≤10% positive); (B) grade 1 (11% to 25% positive); (C) grade 2 (26% to 50% positive); (D) grade 3 (≥51% positive). |
 | Fig. 2Survival analysis according to the epidermal growth factor-like domain 7 (EGFL7) expression (low EGFL7 expression [grade 0 or 1] vs. high EGFL7 expression [grade 2 or 3]). Kaplan-Meier analysis showed that the patients with high EGFL7 expression had a poorer disease-free survival than those with low EGFL7 expression. |
Table 1
Relationship between EGFL7 expression and clinicopathologic characteristics of epithelial ovarian cancer

ACKNOWLEDGEMENT
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu, School of Medicine (2012).
References
1. Park B, Park S, Kim TJ, Ma SH, Kim BG, Kim YM, et al. Epidemiological characteristics of ovarian cancer in Korea. J Gynecol Oncol. 2010; 21:241–247.
2. Li JJ, Yang XM, Wang SH, Tang QL. Prognostic role of epidermal growth factor-like domain 7 protein expression in laryngeal squamous cell carcinoma. J Laryngol Otol. 2011; 125:1152–1157.
3. Huang CH, Li XJ, Zhou YZ, Luo Y, Li C, Yuan XR. Expression and clinical significance of EGFL7 in malignant glioma. J Cancer Res Clin Oncol. 2010; 136:1737–1743.
4. Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009; 50:1839–1850.
5. Fan C, Yang LY, Wu F, Tao YM, Liu LS, Zhang JF, et al. The expression of Egfl7 in human normal tissues and epithelial tumors. Int J Biol Markers. 2013; 28:71–83.
6. Diaz R, Silva J, Garcia JM, Lorenzo Y, Garcia V, Pena C, et al. Deregulated expression of miR-106a predicts survival in human colon cancer patients. Genes Chromosomes Cancer. 2008; 47:794–802.
7. Sun Y, Bai Y, Zhang F, Wang Y, Guo Y, Guo L. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun. 2010; 391:1483–1489.
8. Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, et al. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011; 71:7176–7186.
9. Nomura H, Tamada Y, Miyagi T, Suzuki A, Taira M, Suzuki N, et al. Expression of NEU3 (plasma membrane-associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncol Res. 2006; 16:289–297.
10. Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2012; 119:1345–1352.
11. Meister J, Schmidt MH. miR-126 and miR-126*: new players in cancer. ScientificWorldJournal. 2010; 10:2090–2100.
12. Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, et al. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003; 22:5700–5711.
13. Nikolic I, Plate KH, Schmidt MH. EGFL7 meets miRNA-126: an angiogenesis alliance. J Angiogenes Res. 2010; 2:9.
14. Campagnolo L, Moscatelli I, Pellegrini M, Siracusa G, Stuhlmann H. Expression of EGFL7 in primordial germ cells and in adult ovaries and testes. Gene Expr Patterns. 2008; 8:389–396.
15. Philippin-Lauridant G, Baranzelli MC, Samson C, Fournier C, Pinte S, Mattot V, et al. Expression of Egfl7 correlates with low-grade invasive lesions in human breast cancer. Int J Oncol. 2013; 42:1367–1375.
16. Johnson L, Huseni M, Smyczek T, Lima A, Yeung S, Cheng JH, et al. Anti-EGFL7 antibodies enhance stress-induced endothelial cell death and anti-VEGF efficacy. J Clin Invest. 2013; 123:3997–4009.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download