Abstract
Objective
According to the International Federation of Gynecology and Obstetrics staging, some surgeons perform lymphadenectomy in all patients with early stage endometrial cancer to enable the accurate staging. However, there are some risks to lymphadenectomy such as lower limb lymphedema. The aim of this study was to investigate whether preoperative assessment is useful to select the patients in whom lymphadenectomy can be safely omitted.
Methods
We evaluated the risk of lymph node metastasis (LNM) using LNM score (histological grade, tumor volume measured in magnetic resonance imaging [MRI], and serum CA-125), myometrial invasion and extrautrerine spread assessed by MRI. Fifty-six patients of which LNM score was 0 and myometrial invasion was less than 50% were consecutively enrolled in the study in which a lymphadenectomy was initially intended not to perform. We analyzed several histological findings and investigated the recurrence rate and overall survival.
Results
Fifty-one patients underwent surgery without lymphadenectomy. Five (8.9%) who had obvious myometrial invasion intraoperatively underwent systematic lymphadenectomy. One (1.8%) with endometrial cancer which was considered to arise from adenomyosis had para-aortic LNM. Negative predictive value of deep myometrial invasion was 96.4% (54/56). During the mean follow-up period of 55 months, one patient with deep myometrial invasion who refused an adjuvant therapy had tumor recurrence. The overall survival rate was 100% during the study period.
The International Federation of Gynecology and Obstetrics (FIGO) recommended in 1988 that adequate surgical staging requires a total abdominal hysterectomy, bilateral salpingo-oophorectomy including pelvic and para-aortic lymphadenectomy [1]. New revised 2008 staging system subdivided node-positive stage IIIC disease into two categories, i.e., IIIC1 and IIIC2 were established for the patients with positive pelvic nodes and with positive para-aortic lymph nodes, respectively [2]. According to the FIGO staging, some surgeons believe that lymphadenectomy should be performed in all patients to enable the accurate staging and to assess the necessity for postoperative treatment. Indeed, pelvic and para-aortic lymphadenectomy have been performed in many institutions in Asia (approximately 67% to 98% and 43% to 93%, respectively) [3,4]. However, there are some risks to lymphadenectomy such as postoperative deep vein thrombosis or lower limb lymphedema that may impair the patients' quality of life. Current risk classification in endometrial cancer is based on post-operative final pathological findings including myometrial invasion, histological grade, lymphovascular space invasion (LVSI) and lymph node metastasis (LNM). Thus, one of the most important issues for endometrial cancer is to establish a standard method to predict LNM preoperatively according to the risk evaluation criteria.
We have previously reported that volume index by magnetic resonance imaging (MRI) representing tumor volume, preoperative histological examination (histological subtype and histological grade) and preoperative serum CA-125 level are independent predictors for LNM, and combination of these three risk factors could estimate the risk of LNM in endometrial cancer (LNM score) in different cohorts [5,6]. Myometrial invasion with preoperative MRI was shown not to be an independent risk factor for LNM in our previous studies [5,6]. However, myometrial invasion has been widely considered as one of the most important risk factors for LNM [7]. Thus, we have consecutively performed primary surgery without lymphadenectomy for endometrial cancer patients who met all of the following criteria assessable preoperatively: (1) LNM score was zero, (2) no definitive myometrial invasion and extrauterine spread by MRI. In this study, we aimed to examine whether lymphadenectomy can be safely omitted for patients without risk of LNM evaluated based on preoperative assessments.
A total of 262 patients with endometrial cancer underwent primary surgical treatment from 2003 to 2013 at Hokkaido University Hospital, Sapporo, Japan. Among them, the patients in whom LNM score was 0, myometrial invasion evaluated by MRI was ≤1/2, and no extrauterine spread by MRI, gave their informed consent to omit lymphadenectomy and they were enrolled in this analysis under Institutional Review Board-approval (register ID 013-0092).
LNM score was determined as previously described [5]. Briefly, volume index representing tumor volume was evaluated in either of T2-weighted MRIs or gadolinium-enhanced T1-weighted images, which show tumor lesions more clearly. Volume index was defined as the product of the maximum longitudinal diameter along the uterine axis, the maximum anteroposterior diameter (thickness) in a sagittal section image, and the maximum horizontal diameter in a horizontal section image and the upper limit of volume index (36 cm3) [6]. Myometrial invasion was categorized into three levels: (1) no invasion when a clear junctional zone could be identified in a T2-weighted image and when the border between the endometrium and the myometrium was smooth and clear; (2) invasion of less than one half the myometrium when a partially ruptured junctional zone was identified or when the border between the endometrium and the myometrium was irregular, with tumor signals remaining in one half of the myometrium; and (3) invasion of more than one half the myometrium when a partially interrupted junctional zone was identified or when the border between the endometrium and myometrium was irregular, with tumor signals in more than one half the myometrium. The serum CA-125 level was determined with a chemiluminescent immune assay kit (Abbott Japan, Tokyo, Japan). Two cutoff values (28 U/mL for patients aged >50 years and 70 U/mL for patients aged ≤50 years) divided patients into low and high CA-125 groups for pelvic LMN as previously described [6]. Preoperative endometrial biopsy specimens were evaluated for histological type and histological grade. Frozen section of the resected uterus was carried out to evaluate myometrial invasion according to the surgeons' preference during surgery.
Postoperative pathologic specimens were evaluated for histological type, histological grade (three grades according to the 1988 FIGO criteria), myometrial invasion (no invasion, invasion of less than one half of the myometrium, or invasion of one half or more than half of the myometrium), LVSI (absent or present), cervical invasion (negative or positive stromal invasion), ovarian metastasis (absent or present). Lymph node involvement was assessed in patients undergoing systematic pelvic and para-aortic lymphadenectomy.
Among 262 patients, LNM score was 0 in 71 patients (27%). Thirty two patients (12%) whose LNM score were not 0 did not undergo lymphadenectomy because of various reasons such as high age and severe complications and they are excluded in this analysis. No patients with LNM score 0 refused to consent.
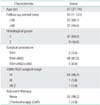
A total of 56 patients were consecutively treated surgically without lymphadenectomy. Patients' characteristics are shown in Table 1. There were four patients under 40 years old and two of them underwent hysterectomy without bilateral salpingo-oophorectomy. All patients were diagnosed as endometrioid adenocarcinoma and no grade 3 tumors were found. Peritoneal washing cytology was carried out for all patients. Lymphadenectomy was not performed in 51 patients because they did not show any definitive myometrial invasion by the macroscopic and/or microscopic inspection of resected uterus during surgery. Frozen section of the resected uterus to evaluate myometrial invasion during surgery was carried out for 23 patients (41.2%, 23/56). Five patients underwent systematic pelvic and para-aortic lymphadenectomy because intraoperative assessment by the frozen section of the resected uterus showed definitive myometrial invasion.
Fifty-four patients were categorized as FIGO 2008 stage IA (pT1ANXM0) and there was one patient with stage IB (pT1BNXM0) and one patient with stage IIIC2 (pT3ApN1M0). Peritoneal washing cytology was negative in all patients. One case with IIIC2 received adjuvant chemotherapy of CAP (cyclophosphamide, 350 mg/m2; adriamycin, 40 mg/m2; and cisplatin, 50 to 70 mg/m2) every 3 weeks for six cycles.
Overall, the tumor volume was relatively small. Seventeen patients (30.4%) had minimal myometrial invasion less than inner half of the uterine corpus and other 39 patients had no myometrial invasion (Table 2). There were no findings that suggested cervical stromal invasion by MRI or extrauterine spread by computed tomography in all patients.
All patients were diagnosed as endometrioid adenocarcinoma and no grade 3 tumors were found. In all 51 patients without lymphadenectomy, no swelling of lymph nodes was palpable during surgery. In five patients with lymphadenectomy, four showed negative nodes and only one patient had both positive nodes and ovarian metastasis (a diameter of 3 mm) (Table 3). Notably, metastatic node was found in para-aortic area above inferior mesenteric artery without positive pelvic node involvement.
The accuracy of histological grade was 89.4% in grade 1 (42/47, five patients who were diagnosed as grade 1 were finally diagnosed as grade 2) and 33.3% in grade 2 (3/9, six patients who were diagnosed as grade 1 were finally diagnosed as grade 2), respectively. The negative predictive value of deep myometrial invasion had an accuracy of 96.4% (54/56) and two patients (3.6%) showed deep myometrial invasion by final pathological examination.
The 98.2% (55/56) of the patients did not show any evidence of recurrent disease after 10 months from final registration. Since one patient with stage IB, who showed deep myometrial invasion and refused adjuvant therapy, resulted in disease recurrence in the vaginal stump at 30 months after surgery, she received radiotherapy for the recurrent tumor and is alive with disease. No lymphatic failure was observed in this patients' cohort. The 3-year and 5-year recurrence free survival rate was 97.3% and 96%, respectively, and the one with recurrent disease was the patient who refused adjuvant therapy even though deep myometrial invasion was confirmed in the permanent specimen. The estimated 5-year overall survival rate was 100% during study period.
Lymphadenectomy is currently one of the most controversial discussed topics in the management of endometrial cancer. Since FIGO introduced surgical staging of endometrial cancer in 1988, essential questions have remained unanswered including the extent of an optimal lymphadenectomy and which subgroup of patients would benefit. We have routinely performed systematic pelvic and para-aortic lymphadenectomy up to the level of renal vein for endometrial cancer in our institute [8,9] and recently demonstrated that para-aortic lymphadenectomy has survival effect on endometrial cancer patients with postoperative intermediate-risk/high-risk for recurrence [10]. On the other hand, it has been established that patients with low-risk for recurrence would not benefit by lymphadenectomy based on the results shown in several studies [10,11,12].
Korean Gynecologic Oncology Group recently proposed the prediction model using combination of (1) preoperative serum CA-125 value, (2) preoperative histological grade, (3) preoperative indirect evaluation of myometrial invasion by MRI, and (4) preoperative evaluation of lymph node swelling and extrauterine spread by MRI, and conclude that their prediction model is useful to select patients with low-risk for LNM preoperatively [13,14]. Mariani et al. [15] defined patients with endometrioid type, grade 1 or 2 tumor, myometrial invasion ≤50%, and no intraoperative evidence of macroscopic extrauterine spread as low-risk corpus cancer, and such patients could be treated optimally with hysterectomy only.
LNM score can discriminate approximately 50% of endometrial cancer patients as low-risk for para-aortic LNM since para-aortic LNM was found in less than 1% in those patients, who do not receive therapeutic benefit with para-aortic lymphadenectomy [6]. However, because patients with LNM score zero included some patients with deep myometrial invasion, who showed 6.8% of positive pelvic LNM [6], LNM score alone may not be sufficient to select patients with low-risk for pelvic LNM. To more accurately select patients with low-risk for pelvic LNM preoperatively, combination of LNM score and myometrial invasion with preoperative MRI was consecutively utilized in this study, because such combination demonstrated that LNM score zero with myometrial invasion less than half revealed approximately 3% of pelvic LNM [6], which seems clinically acceptable to omit lymphadenectomy itself.
Current study showed that high concordance rate of myometrial invasion (<1/2 or ≥1/2) between preoperative and postoperative assessment. There were 16 patients with less than half myometrial invasion which was observed in preoperative MRI and the histological invasion less than half of the myometrium was observed in 15 patient of them (93.8%). From these results, tumor volume measured in MRI could be as useful to predict the extent of myometrial invasion depth as the traditional index such as junctional zone assessment in the patients who might have a myometrial invasion by preoperative MRI. However, there is a considerable restriction about tumor volume measurement in the patients with polypoid tumor. Additionally, one patient in this study with endometrial cancer that arose from the widespread adenomyosis showed a positive para-aortic node and such cases may be out of this assessment.
Although this is a retrospective small-scale study, we enrolled the consecutive patients in the study period. In this study, we demonstrated that the prognosis of the patients in whom lymphadenectomy was omitted according to our preoperative assessments by the combination of LNM score, evaluation of myometrial invasion and extrauterine spread by MRI was quite favorable. Our criteria may also be useful to estimate the prognosis of patients in whom aggressive operative procedures including sentinel lymph node biopsy cannot be tolerated because of unavoidable reasons such as severe complications and advanced age [16]. It should be noted that our study did not address the issue of routine lymphadenectomy for the high-risk groups. The benefit of lymphadenectomy in this risk group should be assessed by further randomized clinical trials.
In conclusion, current study confirmed that preoperative assessments by the combination of LNM score with evaluation of myometrial invasion and extrauterine spread with MRI is useful to select the patients without risk of LNM and to safely omit lymphadenectomy. Unlike previous risk models that were based on final pathologic findings [7,17], our risk criteria might be useful in designing clinical trials. The reproducibility of these predictors and the predictive accuracy should be validated prospectively in the future clinical trials.
Figures and Tables
Table 1
Characteristics of endometrial cancer patients with potential low-risk for lymph node metastasis (n=56)
References
1. Announcements: FIGO stages 1988 revision. Gynecol Oncol. 1989; 35:125–127.
2. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009; 105:103–104.
3. Lee TS, Kim JW, Kim SH, Seong SJ, Song ES, Kim JH, et al. Surgical practice patterns in endometrial cancer: results of the Korean Gynecologic Oncology Group survey. J Gynecol Oncol. 2009; 20:107–112.
4. Watanabe Y, Aoki D, Kitagawa R, Takeuchi S, Sagae S, Sakuragi N, et al. Status of surgical treatment procedures for endometrial cancer in Japan: results of a Japanese Gynecologic Oncology Group survey. Gynecol Oncol. 2007; 105:325–328.
5. Todo Y, Sakuragi N, Nishida R, Yamada T, Ebina Y, Yamamoto R, et al. Combined use of magnetic resonance imaging, CA 125 assay, histologic type, and histologic grade in the prediction of lymph node metastasis in endometrial carcinoma. Am J Obstet Gynecol. 2003; 188:1265–1272.
6. Todo Y, Okamoto K, Hayashi M, Minobe S, Nomura E, Hareyama H, et al. A validation study of a scoring system to estimate the risk of lymph node metastasis for patients with endometrial cancer for tailoring the indication of lymphadenectomy. Gynecol Oncol. 2007; 104:623–628.
7. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer: a Gynecologic Oncology Group Study. Cancer. 1987; 60:8 Suppl. 2035–2041.
8. Watari H, Todo Y, Takeda M, Ebina Y, Yamamoto R, Sakuragi N. Lymph-vascular space invasion and number of positive para-aortic node groups predict survival in node-positive patients with endometrial cancer. Gynecol Oncol. 2005; 96:651–657.
9. Watari H, Mitamura T, Moriwaki M, Hosaka M, Ohba Y, Sudo S, et al. Survival and failure pattern of patients with endometrial cancer after extensive surgery including systematic pelvic and para-aortic lymphadenectomy followed by adjuvant chemotherapy. Int J Gynecol Cancer. 2009; 19:1585–1590.
10. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010; 375:1165–1172.
11. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100:1707–1716.
12. ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–136.
13. Lee JY, Jung DC, Park SH, Lim MC, Seo SS, Park SY, et al. Preoperative prediction model of lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2010; 20:1350–1355.
14. Kang S, Kang WD, Chung HH, Jeong DH, Seo SS, Lee JM, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean gynecologic oncology group study. J Clin Oncol. 2012; 30:1329–1334.
15. Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008; 109:11–18.
16. Ballester M, Dubernard G, Bats AS, Heitz D, Mathevet P, Marret H, et al. Comparison of diagnostic accuracy of frozen section with imprint cytology for intraoperative examination of sentinel lymph node in early-stage endometrial cancer: results of Senti-Endo study. Ann Surg Oncol. 2012; 19:3515–3521.
17. Akbayir O, Corbacioglu A, Goksedef BP, Numanoglu C, Akca A, Guraslan H, et al. The novel criteria for predicting pelvic lymph node metastasis in endometrioid adenocarcinoma of endometrium. Gynecol Oncol. 2012; 125:400–403.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download