Abstract
Objective
A number of new techniques have been developed to prevent lymphocele formation after pelvic lymphadenectomy in gynecologic cancers. We assessed whether the electrothermal bipolar vessel sealing device (EBVSD) could decrease the incidence of postoperative lymphocele secondary to pelvic lymphadenectomy.
Methods
A total of 321 patients with gynecologic cancer underwent pelvic lymphadenectomy from 2005 to 2011. Pelvic lymphadenectomy without EBVSD was performed in 134 patients, and pelvic lymphadenectomy with EBVSD was performed in 187 patients. We retrospectively compared the incidence of lymphocele and symptoms between both groups.
Results
Four to 8 weeks after operation, 108 cases of lymphocele (34%) were detected by computed tomography scan examination. The incidence of lymphocele after pelvic lymphadenectomy was 56% (75/134) in the tie ligation group, and 18% (33/187) in the EBVSD group. We found a statistically significant difference in the incidence of lymphocele between both groups (p<0.01). To detect the independent risk factor for lymphocele development, we performed multivariate analysis with logistic regression for three variables (device, number of dissected lymph nodes, and operation time). Among these variables, we found a significant difference (p<0.001) for only one device.
Pelvic lymphocele, one of the sequelae of pelvic lymphadenectomy, is defined as a collection of lymphatic fluid without distinct epithelial lining, resulting from the transection of afferent lymphatic channels [1]. Lymphocele is caused by leakage of lymph from afferent lymphatic channels as the result of tissue trauma or operation. Pelvic lymphadenectomy is a crucial step in gynecologic cancer operation. The most frequent postoperative complication of pelvic lymphadenectomy is lymphocele, also known as lymphocyst, and it is a consequence of surgical dissection and inadequate closure of afferent lymphatic vessels. In literature, the reported incidences of clinically detected lymphocele after pelvic lymphadenectomy range from 1% to 49% [2,3,4,5,6,7,8,9,10,11]. The risk factors of lymphocele include extensive pelvic lymphadenectomy, number of lymph nodes (LNs) removed, lack of ligation of lymphatic vessels, preoperative or postoperative radiation therapy, presence of metastasis to the LNs, use of retroperitoneal suction drainage, and administration of low-dose heparin for thromboembolic prophylaxis [12,13].
Most lymphoceles are asymptomatic; thus, they are found incidentally. However, large lymphoceles may sometimes be symptomatic, resulting from compression of surrounding structures. Associated symptoms include pelvic pain, leg edema and pain, hydronephrosis, and deep vein thrombosis (DVT). Furthermore, if the lymphocele becomes infected, an abscess may form, and possibly cause sepsis.
To prevent postoperative lymphocele formation, a number of techniques have been challenged so far, including the nonclosure of the pelvic peritoneum, absence of retroperitoneal drainage, omentoplasty, and fibrin application. Although these techniques have been developed, little has been reported on whether they lead to a significant reduction in postoperative lymphocele after pelvic lymphadenectomy [7,8,10,11,14,15,16,17,18].
The electrothermal bipolar vessel sealing device (EBVSD) has been designed to aid in coagulation and dissection with less thermal spread than conventional electrocautery. We introduced EBVSD to gynecologic cancer operation in 2007. Considering the ability of this method to firmly seal the lymphatic vessels, we hypothesized that EBVSD could decrease the incidence of postoperative lymphocele secondary to pelvic lymphadenectomy. To our knowledge, there are only a few up-to-date studies focusing on lymphocele development after the use of EBVSD post pelvic lymphadenectomy in patients with gynecologic cancers. The aim of this study was to clarify whether EBVSD contributed to a decrease in the incidence of postoperative lymphocele secondary to pelvic lymphadenectomy.
A total of 321 patients with gynecologic cancer underwent surgical procedures including pelvic lymphadenectomy, at the Department of Obstetrics and Gynecology of Kurume University Hospital, between 2005 and 2011. These surgeries were performed on patients with cervical cancer (n=126), endometrial cancer (n=119), ovarian cancer (n=70), and other types of gynecologic cancers (n=6). Pelvic lymphadenectomy was performed with total abdominal hysterectomy, radical hysterectomy, or modified radical hysterectomy, with para-aortic LN sampling, with or without bilateral salpingo-oophorectomy.
We did a retrospective analysis of the incidence of lymphocele after pelvic lymphadenectomy, with or without EBVSD in patients with gynecologic cancer. Patients were classified into two groups; the tie ligation and EBVSD groups. Respectively, these groups were compared for each factor, i.e., primary lesion, International Federation of Gynecology and Obstetrics (FIGO) stage, age, bleeding during operation, operation time, number of dissected LNs, LN metastasis, adjuvant radiotherapy, lymphocele formation, and diameter of lymphocele.
Since 2007, we have used the EBVSD (BiClamp, Elektromedizin GmbH, Tubingen, Germany; LigaSure, Covidien, Boulder, CO, USA) in the operation of gynecologic cancer.
Pelvic lymphadenectomy was defined as the excision of all fibro-fatty tissue along the external iliac vein, including the bifurcation of the common iliac artery together with fibro-fatty tissue within the obturator fossa. During pelvic lymphadenectomy, we paid special attention to seal or ligate lymphatic vessels: (1) at the level of the femoral canal, on the ventral walls of external iliac vessels; (2) at the level of obturator fossa, where numerous channels are connected with the lateral parametria; and (3) at the bifurcation of common iliac vessels, cranially to the internal iliac vessels, and medial to the external iliac vessels (Fig. 1).
In this series, we did not suture the retroperitoneum in all patients. From 2005 to 2008, a Penrose drain was placed in each paravesical space and led outward through the vagina. Since 2009, a closed suction drain was placed through the abdominal wall into each paravesical space. The volume of fluid from each drain was measured daily. The drains were removed when the fluid drainage was <100 mL/day. All patients received intraoperative antibiotic prophylaxis, which was continued postoperatively for 3 days. The dissected LNs were counted and submitted for histopathological examination.
We checked for lymphocele by computed tomography (CT) between 4 and 8 weeks postoperatively. We also checked routinely for lymphocele and recurrent disease by CT at 6 months postoperatively. In the event that lymphocele was detected during postoperative visits, we started close follow-up by CT or ultrasonography. All findings were checked by a radiologist. The finding of a smooth and thin-walled cavity filled with a water-equivalent fluid, which was sharply demarcated from its surroundings on CT, was interpreted as lymphocele. In this series, we diagnosed a symptomatic lymphocele if the patient had at least one grade 1, 2, or 3 adverse effects according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. Main symptoms were lower abdominal pain, back pain, prolonged ileus, leg pain, edema, or fever. We also retrospectively compared the incidence and diameter of symptomatic lymphocele between groups: the tie ligation and the EBVSD groups.
For parametric analyses, we used the Student t-test and Welch t-test, while for nonparametric analyses we used the Mann-Whitney U-test. Statistical analysis was performed with the chi-square test in a contingency table. Simultaneous analysis of all possible influential variables was performed with logistic regression. A p<0.05 was regarded as statistically significant. The data collected were analyzed using JMP ver. 8.0.2 (SAS Institute Inc., Cary, NC, USA).
Three hundred twenty-one patients underwent pelvic lymphadenectomy in Kurume University Hospital between 2005 and 2011. From 2005 to 2007, 134 patients underwent pelvic lymphadenectomy without EBVSD (tie ligation group). From 2007 to 2011, 187 patients had pelvic lymphadenectomy with EBVSD (EBVSD group). Comparison of patient characteristics between the two groups was shown in Table 1. Between the two groups, there were no statistically significant differences in bleeding during the operation, number of dissected pelvic LNs, LN metastasis, or adjuvant radiotherapy. The operation time was longer in the EBVSD group (p=0.014) (Table 2).
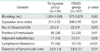
Four to 8 weeks after operations, in 108 of the 315 patients, lymphocele (34%) was detected by CT scan. The incidence of lymphocele after pelvic lymphadenectomy was 56% (75/134) in the tie ligation group, while only 18% (33/187) in the EBVSD group. We found a notable decrease in the incidence of lymphocele in the EBVSD group (p<0.01). The mean±SD diameter of the lymphocele was 3.8±1.6 cm in the tie ligation group, and 3.8±2.1 cm in the EBVSD group. There were no significant differences between both groups (Table 2).
The total incidence of symptomatic lymphocele was 9% (29/321), while asymptomatic lymphocele was 25% (79/321). We found that the incidence of symptoms was in proportion to the diameter of the lymphocele. The mean±SD diameter of lymphoceles was 5.2±2.0 cm in symptomatic lymphoceles, and 3.3±1.3 cm in asymptomatic lymphoceles (p<0.01). The main presenting symptoms were lower abdominal pain, fever, and ileus, which developed from 2 to 6 weeks after the operation. Symptomatic lymphoceles included 21% (6/29) with grade 1 adverse effect (light low abdominal pain without fever), and 79% (23/29) with grades 2 and 3 adverse effect (fever, pelvic abscess, ileus, or hydronephrosis) in CTCAE ver. 4.0.
A notable difference (p<0.001) in the incidence of symptomatic lymphocele was found between the tie ligation group (14%, 19/134) and the EBVSD group (5.3%, 10/187). However, in terms of the ratio of the two groups in 108 cases with lymphocele, there was no statistical difference in the incidence of symptomatic lymphocele (tie ligation group: 25%, 19/75; EBVSD group: 30%, 10/33; p=0.59). All lymphocele diminished within ten months without surgical treatment. In the EBVSD group, there was no difference in the postoperative drainage volume between a closed suction drain and a Penrose drain (open; p=0.9).
We performed univariate analysis of all possible influential variables that could influence the development of lymphocele (device [tie ligation vs. EBVSD], primary lesion, FIGO stage, age [<50 vs. ≥50], bleeding, operation time, number dissected of LNs, LN metastasis, and adjuvant radiotherapy) (Table 3). There was significant difference in device (p<0.01). Additionally, there seemed to be some difference in operation time (p=0.071) and number dissected of LNs (p=0.10). On the other hand, there were no significant difference in primary lesion, FIGO stage, age, bleeding, LN metastasis, and adjuvant radiotherapy. Furthermore, we performed multivariate analysis with logistic regression on three variables (device, operation time, and number dissected of LNs). We found that there was only a significant difference for device (p<0.001) among these variables. A high odds ratio (OR) 5.61 was observed in the tie ligation group, while a low OR 0.18 was found in the EBVSD group (Table 3).
The presentation of lymphocele may be quite diverse in pelvic cancer operation. The rate of asymptomatic lymphocele may approach 50% to 60% [19]. Symptomatic patients may present with ileus, pain, fever, lower extremity edema, or urinary complaints [20]. Symptomatic or clinically significant lymphocele was reported in 1.6% to 3.5% of patients who underwent pelvic lymphadenectomy for prostate cancer [21,22]. In our research, we found that 34% of patients with gynecologic cancer undergoing pelvic lymphadenectomy at our hospital between 2005 and 2011 developed lymphocele.
The formation of lymphocele may be promoted by several factors: extensive pelvic lymphadenectomy, the number of LNs removed, lack of ligation of lymph vessels, the presence of metastasis to LNs, postoperative radiation therapy, the presence of drainage, and the prophylactic use of anticoagulants [1,7,8,10,11,13,14,15,16,17,18,23,24,25,26,27,28]. However, none of these potential factors has been proven to be significant.
A retrospective study reported the risk factors of lymphocele in 264 patients who underwent pelvic lymphadenectomy during gynecologic cancer operations [29]. Among these patients, body mass index and number of resected LNs were higher in the lymphocele group, and the use of postoperative radiotherapy was associated with a higher risk of lymphocele. In the present series, we could not detect a significant difference in the effect of number of dissected of LNs and adjuvant radiotherapy on the formation of lymphocele. In terms of extensive lymphadenectomy, patients in this series underwent pelvic lymphadenectomy with para-aortic LN sampling. Therefore, we did not check the influence of para-aortic LN dissection on the formation of lymphocele in this series. A complete understanding of the formation of lymphocele remains to be elucidated.
Lymphocele incidence can be minimized by meticulous surgical techniques and attention to sealing the lymphatic vessels during node dissection, by blocking lymphatic drainage from lower extremities and preventing lymph accumulation in the pelvic cavity. In particular, treatment-associated morbidity can be reduced when all lymphatic channels lateral to the external artery are saved or clamped [5,7,8,15]. In the reported series, different techniques for the prevention of lymphocele have been described [14,16,17,18]. The intraoperative application of fibrin glue did not reduce the rate of lymphocele after pelvic lymphadenectomy [18]. Moreover, closed suction drainage or omental flaps have been proposed to prevent the occurrence of pelvic lymphadenectomy-related complications. However, a widely agreed-upon solution is yet to be found [9,14,15,17].
The development of lymphocele is an issue for patients when it leads to sequelae relevant to their health. Additionally to secondary infection of lymphocele, sequelae comprise mainly thromboembolic events due to compression of pelvic vessels. Development of DVT secondary to lymphocele has been reported [30,31]. The reported rate of DVT in patients with gynecologic cancer ranges from 11% to 18%, with a rate of pulmonary embolism (PE) between 1% and 2.6%. Among patients with ovarian cancer, the postoperative rate of PE is as high as 7.2% [24,32,33,34,35,36]. Fortunately, these events are rare in association with pelvic lymphadenectomy, though they represent a significant cause of operative and perioperative mortality.
In this series, we detected five cases of DVT preoperatively. All the patients with DVT were administered intravenous heparin and put on an inferior vena cava (IVC) filter preoperatively. These patients also received intravenous heparin postoperatively until removal of the IVC filter. None of the patients developed DVT postoperatively.
Since 2007, we introduced EBVSD for sealing lymphatic vessels during pelvic lymphadenectomy, in order to prevent the development of lymphocele postoperatively. The introduction of energy-based vessel sealing technologies has expanded the arsenal of potential techniques available for transoperative hemostasis. These devices allow a rapid sequential tissue and vessel sealing, coagulation, and transection. There are several EBVSD currently in use. In our series, we used the bipolar sealing devices LigaSure and BiClamp. LigaSure employs a unique combination of pressure and energy to create vessel fusion. This optimized combination of pressure and energy melts the collagen and elastin in the vessel walls, reforming it into a permanent, plastic-like seal [37]. LigaSure has the highest burst pressure and fastest sealing time compared to other similar devices, and it was the highest rated overall [38]. In this series, we mainly used LigaSure small jaw, which has a suitably sized body (18.8 cm) for lymph vessel sealing and has an appropriate angled jaw (28°) for pelvic floor procedures.
Due to the lack of smooth muscle cells in the wall of lymphatic vessels, a low concentration of clotting factors, and a lack of thrombocytes in lymphatic fluids, it is possible that an EBVSD is less effective in sealing only the lymphatic vessels. Therefore, it is recommended to seal surrounding connective tissue together with the lymphatic vessels to reinforce the sealing effect (Fig. 1). Additionally, we performed double sealing of lymphatic vessel edges, especially the three major lymphatic channels 1 to 3 as mentioned in the Materials and Methods. These ingenuities may contribute to a decrease in the lymphocele development compared with previous reports [2,3,4,5,6,7,8,9,10,11].
Before introducing EBVSD, the prevention of lymphocele formation was achieved by simply tie-ligating the edge of lymphatic vessels. Using only tie ligation, we experienced lymphocele in 56% (75/134) of cases between 2005 and 2007. However, since the introduction of EBVSD on 2007, the incidence of lymphocele dramatically decreased to 18% (33/187). Our findings suggest that the development of lymphocele notably decreases by using EBVSD in pelvic lymphadenectomy.
Symptomatic lymphocele led to considerable delays in the introduction of adjuvant chemotherapy. In the case of patients needing adjuvant chemotherapy, symptomatic lymphocele can account for a significant amount of morbidity and cost because these patients are more susceptible to repeated infections by the bone marrow suppression caused by adjuvant chemotherapy.
In our series, all patients who had grade 2 and 3 adverse effects of symptomatic lymphocele (n=23) were administered intravenous antibiotics for 3 to 5 days. All these patients responded adequately to antibiotic treatment alone, and we did not enforce other treatment strategies, such as percutaneous needle aspiration, percutaneous catheter drainage, sclerotherapy or open drainage by laparotomy, or laparoscopy.
However, in 93% of patients (14/15) with symptomatic lymphocele who had indication of adjuvant chemotherapy, the induction of first cycle of chemotherapy was delayed. Considering these delays, especially for patients who need adjuvant chemotherapy, we may suggest attempting other treatment strategies, such as percutaneous needle aspiration in addition to the administration of intravenous antibiotics.
The difference in the size of lymphocele between the tie ligation group and the EBVSD group was not discernable. Compared with the tie ligation group, a considerable amount of diminutive cases of symptomatic lymphocele formation were observed in the EBVSD group, while there was no difference in the ratio of symptomatic to asymptomatic lymphocele between groups. Therefore, the introduction of EBVSD could decrease the development of symptomatic lymphocele by decreasing the total number of lymphocele themselves.
The limitation of this study was its retrospective design. Therefore, further prospective studies of technique comparisons, such as EBVSD versus tie ligation versus clip, are needed to improve the selection process of proper devices to prevent lymphocele formation.
In conclusion, the use of EBVSD during pelvic lymphadenectomy could be beneficial in the prevention of lymphocele development in patients with gynecological cancer.
Figures and Tables
 | Fig. 1Boxed parts indicate: (1) the level of the femoral canal, on the ventral walls of external iliac vessels; (2) the level of obturator fossa, where numerous channels are connected with the lateral parametria; and (3) the bifurcation of common iliac vessels, cranially to the internal iliac vessels, and medial to the external iliac vessels. We performed double sealing of the lymphatic vessel edge at the level of the obturator fossa by LigaSure small jaw. |
Table 1
Comparison of patient characteristics between tie ligation and electrothermal bipolar vessel sealing device groups
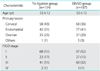
Table 2
Comparison of treatment characteristics between tie ligation and electrothermal bipolar vessel sealing device groups
References
1. Rutledge F, Dodd GD Jr, Kasilag FB Jr. Lymphocysts: a complication of radical pelvic surgery. Am J Obstet Gynecol. 1959; 77:1165–1175.
2. Querleu D, Leblanc E, Cartron G, Narducci F, Ferron G, Martel P. Audit of preoperative and early complications of laparoscopic lymph node dissection in 1000 gynecologic cancer patients. Am J Obstet Gynecol. 2006; 195:1287–1292.
3. Kohler C, Klemm P, Schau A, Possover M, Krause N, Tozzi R, et al. Introduction of transperitoneal lymphadenectomy in a gynecologic oncology center: analysis of 650 laparoscopic pelvic and/or paraaortic transperitoneal lymphadenectomies. Gynecol Oncol. 2004; 95:52–61.
4. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009; 27:5331–5336.
5. Ilancheran A, Monaghan JM. Pelvic lymphocyst: a 10-year experience. Gynecol Oncol. 1988; 29:333–336.
6. Tam KF, Lam KW, Chan KK, Ngan HY. Natural history of pelvic lymphocysts as observed by ultrasonography after bilateral pelvic lymphadenectomy. Ultrasound Obstet Gynecol. 2008; 32:87–90.
7. Conte M, Panici PB, Guariglia L, Scambia G, Greggi S, Mancuso S. Pelvic lymphocele following radical para-aortic and pelvic lymphadenectomy for cervical carcinoma: incidence rate and percutaneous management. Obstet Gynecol. 1990; 76:268–271.
8. Benedetti-Panici P, Maneschi F, Cutillo G, D'Andrea G, di Palumbo VS, Conte M, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecol Oncol. 1997; 65:478–482.
9. Mann WJ, Vogel F, Patsner B, Chalas E. Management of lymphocysts after radical gynecologic surgery. Gynecol Oncol. 1989; 33:248–250.
10. Patsner B. Closed-suction drainage versus no drainage following radical abdominal hysterectomy with pelvic lymphadenectomy for stage IB cervical cancer. Gynecol Oncol. 1995; 57:232–234.
11. Panici PB, Plotti F, Zullo MA, Muzii L, Manci N, Palaia I, et al. Pelvic lymphadenectomy for cervical carcinoma: laparotomy extraperitoneal, transperitoneal or laparoscopic approach? A randomized study. Gynecol Oncol. 2006; 103:859–864.
12. Symmonds RE, Pratt JH. Prevention of fistulas and lymphocysts in radical hysterectomy: preliminary report of a new technic. Obstet Gynecol. 1961; 17:57–64.
13. Catalona WJ, Kadmon D, Crane DB. Effect of mini-dose heparin on lymphocele formation following extraperitoneal pelvic lymphadenectomy. J Urol. 1980; 123:890–892.
14. Clarke-Pearson DL, Synan IS, Creasman WT. Significant venous thromboembolism caused by pelvic lymphocysts: diagnosis and management. Gynecol Oncol. 1982; 13:136–143.
15. Yamamoto R, Saitoh T, Kusaka T, Todo Y, Takeda M, Okamoto K, et al. Prevention of lymphocyst formation following systematic lymphadenectomy. Jpn J Clin Oncol. 2000; 30:397–400.
16. Fujiwara K, Kigawa J, Hasegawa K, Nishimura R, Umezaki N, Ando M, et al. Effect of simple omentoplasty and omentopexy in the prevention of complications after pelvic lymphadenectomy. Int J Gynecol Cancer. 2003; 13:61–66.
17. Logmans A, Kruyt RH, de Bruin HG, Cox PH, Pillay M, Trimbos JB. Lymphedema and lymphocysts following lymphadenectomy may be prevented by omentoplasty: a pilot study. Gynecol Oncol. 1999; 75:323–327.
18. Scholz HS, Petru E, Benedicic C, Haas J, Tamussino K, Winter R. Fibrin application for preventing lymphocysts after retroperitoneal lymphadenectomy in patients with gynecologic malignancies. Gynecol Oncol. 2002; 84:43–46.
19. Solberg A, Angelsen A, Bergan U, Haugen OA, Viset T, Klepp O. Frequency of lymphoceles after open and laparoscopic pelvic lymph node dissection in patients with prostate cancer. Scand J Urol Nephrol. 2003; 37:218–221.
20. Loeb S, Partin AW, Schaeffer EM. Complications of pelvic lymphadenectomy: do the risks outweigh the benefits? Rev Urol. 2010; 12:20–24.
21. Campbell SC, Klein EA, Levin HS, Piedmonte MR. Open pelvic lymph node dissection for prostate cancer: a reassessment. Urology. 1995; 46:352–355.
22. Pepper RJ, Pati J, Kaisary AV. The incidence and treatment of lymphoceles after radical retropubic prostatectomy. BJU Int. 2005; 95:772–775.
23. Pennehouat G, Mosseri V, Durand JC, Hamelin JP, Asselain B, Pilleron JP, et al. Lymphoceles and peritonization following lymphadenectomy for cancer of the uterus. J Gynecol Obstet Biol Reprod (Paris). 1988; 17:373–378.
24. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983; 61:87–94.
25. Orr JW Jr, Barter JF, Kilgore LC, Soong SJ, Shingleton HM, Hatch KD. Closed suction pelvic drainage after radical pelvic surgical procedures. Am J Obstet Gynecol. 1986; 155:867–871.
26. Nelson JH Jr, Huston JW. Lymphocyst formation following pelvic lymphadenectomy. Am J Obstet Gynecol. 1959; 78:1298–1300.
27. de Roo T. The value of lymphography in lymphedema. Surg Gynecol Obstet. 1967; 124:755–765.
28. Ferguson JH, Maclure JG. Lymphocele following lymphadenectomy. Am J Obstet Gynecol. 1961; 82:783–792.
29. Kim HY, Kim JW, Kim SH, Kim YT, Kim JH. An analysis of the risk factors and management of lymphocele after pelvic lymphadenectomy in patients with gynecologic malignancies. Cancer Res Treat. 2004; 36:377–383.
30. Augustin H, Hammerer P, Graefen M, Palisaar J, Noldus J, Fernandez S, et al. Intraoperative and perioperative morbidity of contemporary radical retropubic prostatectomy in a consecutive series of 1243 patients: results of a single center between 1999 and 2002. Eur Urol. 2003; 43:113–118.
31. Heinzer H, Hammerer P, Graefen M, Huland H. Thromboembolic complication rate after radical retropubic prostatectomy. Impact of routine ultrasonography for the detection of pelvic lymphoceles and hematomas. Eur Urol. 1998; 33:86–90.
32. Bakhru A. Effect of ovarian tumor characteristics on venous thromboembolic risk. J Gynecol Oncol. 2013; 24:52–58.
33. Clark-Pearson DL, DeLong E, Synan IS, Soper JT, Creasman WT, Coleman RE. A controlled trial of two low-dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol. 1990; 75:684–689.
34. Martino MA, Borges E, Williamson E, Siegfried S, Cantor AB, Lancaster J, et al. Pulmonary embolism after major abdominal surgery in gynecologic oncology. Obstet Gynecol. 2006; 107:666–671.
35. Musch M, Klevecka V, Roggenbuck U, Kroepfl D. Complications of pelvic lymphadenectomy in 1,380 patients undergoing radical retropubic prostatectomy between 1993 and 2006. J Urol. 2008; 179:923–928.
36. Zorn KC, Katz MH, Bernstein A, Shikanov SA, Brendler CB, Zagaja GP, et al. Pelvic lymphadenectomy during robot-assisted radical prostatectomy: assessing nodal yield, perioperative outcomes, and complications. Urology. 2009; 74:296–302.
37. Kennedy JS, Stranahan PL, Taylor KD, Chandler JG. High-burst-strength, feedback-controlled bipolar vessel sealing. Surg Endosc. 1998; 12:876–878.
38. Lamberton GR, Hsi RS, Jin DH, Lindler TU, Jellison FC, Baldwin DD. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008; 22:2307–2312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download