Abstract
Objective
To compare the efficacy of metformin plus megestrol acetate (MA) with that of MA alone for treating endometrial atypical hyperplasia (EAH).
Methods
This pilot study included 16 EAH patients who met at least one metabolic syndrome (MS) criterion and received either adjunctive metformin plus MA (MET group) or MA monotherapy (MA group). Each patient in the MA group received 160 mg of MA daily, whereas patients in the MET group received the same dose of MA plus 0.5 g of metformin thrice daily. Treatment response was assessed by histological examination of dilation and curettage specimens obtained after 12 weeks of therapy.
Results
Each group had eight patients, and half of the patients in each group were diagnosed with MS. The complete response (CR) rate was 75% (6/8) in the MET group and 25% (2/8) in the MA group (p=0.105). Complications of MS did not affect the response rates in either group. In the MET group, 75% (3/4) of the patients had CR in the presence or absence of MS. In the MA group, 50% (2/4) of the patients with MS had CR, whereas no patient without MS had CR. No irreversible toxicities were observed.
Endometrial atypical hyperplasia (EAH) is a precancerous stage of endometrial cancer (EC). It mostly occurs in postmenopausal women, and surgery is usually the first treatment choice. However, conservative therapy is often demanded by young patients with EAH, who desire to conceive or are unwilling to undergo surgery.
Conservative therapies for EAH patients mainly include the use of progestins such as medroxyprogesterone acetate (MPA) and megestrol acetate (MA). The levonorgestrel intrauterine device, acting through the release of levonorgestrel, has also been demonstrated to be efficacious in the treatment of endometrial hyperplasia and early EC [1,2,3]. The complete response (CR) rate of EAH patients using oral progestin is approximately 70% [1,4,5,6], with the mean response time ranging from 6 to 18 months [7,8]. Moreover, in some studies, a high response rate was achieved by using high doses of progestins, such as MPA at 500 to 1,000 mg/day or MA at 80 to 400 mg/day [5]. High doses of progestins and long treatment periods may affect the compliance of patients and produce unwanted side effects. There is an urgent need for better therapeutic regimens that are more effective in a shorter time.
We found that EAH has an intimate relationship with insulin resistance and metabolic disorder. In addition, a low body mass index of <35 kg/m2 was found to be related to a high resolution rate in EAH patients receiving progestin treatment [7]. Furthermore, case reports show that EAH patients with no response (NR) to progestin therapy could achieve complete reversal when metformin was added [9,10,11]. Moreover, metformin, as an insulin sensitizer, has been found to potentially reduce the incidence of cancer, including liver, pancreas, colorectal, and breast cancer, as well as the mortality of cancer, including liver and breast cancer, in a meta-analysis [12]. In addition, Campagnoli et al. [10] suggested that metformin acts as a preventive agent against EC by inhibiting cancer cell proliferation, and their results also suggested that metformin might be a potent agent for treating EAH. However, clinical evidence of the efficacy of metformin for treating EAH remains unclear. Therefore, we performed a pilot study to examine the efficacy of metformin for treating EAH by comparing the efficacy of metformin plus MA (MET) with that of MA alone in EAH patients.
This was a controlled, single-blinded, prospective cohort study performed between August 2012 and January 2013 on outpatients of the Obstetrics and Gynecology Hospital of Fudan University. In this pilot study, we analyzed only those patients who presented to the hospital during the abovementioned study period. This study was approved by the Ethics Committee of the Obstetrics and Gynecology Hospital of Fudan University. All patients signed informed consent before participating in this research and randomization. The enrolled patients were followed for 3 months.
Patients who were diagnosed with EAH (aged ≤45 years), had a desire for preservation of fertility, and met at least one metabolic syndrome (MS) criterion were enrolled [13,14]. The MS criteria were as follows: (1) elevated waist circumference (WC): WC ≥80 cm for Chinese women; (2) elevated triglycerides (TGs; drug treatment for elevated TGs is an alternative indicator): TGs ≥150 mg/dL; (3) reduced high density lipoprotein cholesterol (HDL-C; drug treatment for reduced HDL-C is an alternative indicator): HDL-C <50 mg/dL in female; (4) elevated blood pressure (antihypertensive drug treatment in a patient with a history of hypertension is an alternative indicator): systolic pressure 130 mm Hg and/or diastolic pressure 85 mm Hg; and (5) elevated fasting blood glucose (FBG; drug treatment for elevated FBG is an alternative indicator): FBG ≥100 mg/dL. In addition, presence of any three of the five risk factors constituted a diagnosis of MS.
All patients were pathologically diagnosed on the basis of the criteria reported in a previous study [15]. Atypical hyperplasia is characterized by cells with nuclear atypia, loss of polarity, and an increase in the nuclear/cytoplasmic ratio. The nuclei are enlarged and irregular in size and shape, with coarse chromatin clumping, a thickened irregular nuclear membrane, and prominent nucleoli.
All patients received endometrial dilation and curettage (D&C) because of menstrual disorder or abnormal vaginal bleeding lasting from a week to several months. Pathological diagnosis of the endometrial curettage samples was confirmed by at least two experienced gynecological pathologists (at least one deputy chief pathologist) at the Obstetrics and Gynecology Hospital of Fudan University. If their opinions differed, a seminar was held in the pathological department for the final diagnosis. Four pretreatment pathological cases from another hospital were also confirmed by at least two experienced gynecological pathologists at our hospital, as described above.
Transvaginal ultrasonography and pelvic examination were performed to exclude the presence of other possible lesions in the reproductive system. The exclusion criteria were alcoholism, pregnancy, severe infection, complicated clinical diseases (dysfunction of the heart, liver, lung, and kidney), severe cardiovascular diseases, allergy history for MA or metformin, thrombosis, or breast cancer history, other malignancies of the reproductive system or EC, and other contraindications of MA or metformin treatment. Previous hormonal treatment was also not allowed.
Data on age, WC, blood pressure, and history of diabetes, hypertension, and thrombus were collected. Blood tests, including TG, HDL-C, FBG, and liver and renal function tests, were performed at 8:00 AM after fasting the night before the start of treatment. Liver function tests included alanine aminotransferase, aspartate aminotransferase, glutamine transaminase, lactate dehydrogenase, alkaline phosphatase, total bilirubin, direct bilirubin, total bile acid, total protein, albumin, and globulin. Renal function tests included urea nitrogen, uric acid, and creatinine. Liver and renal functions were reevaluated after 3 months of treatment. All blood tests were performed using a Hitachi fully automatic biochemical analyzer (Hitachi, Tokyo, Japan), and tests were repeated when the values exceeded the reference values.
Estrogen receptor (ER) and progesterone receptor (PgR) expressions were examined by pathologists in our hospital before and after the therapy, and four patients had no relevant information because they received their pretreatment pathological diagnoses at other hospitals. Immunohistochemical staining was semiquantitatively scored according to the percentage of positive cells: 0, ≤5%; 1+, 6% to 25%; 2+, 26% to 50%; 3+, 51% to 75%; and 4+, 76% to 100% [16].
Adverse effects were recorded during the entire follow-up period, including thrombosis, lactic acidosis, abnormal liver and renal function, and other toxicities or complaints. In addition, relapse following therapy, treatment response, and fertility situation were followed up.
Patients were randomized into two groups. Patients who received 160 mg of oral MA daily were established as controls and are hereafter referred to as the MA group. In the study group, each patient received the same dose of MA plus 0.5 g of oral metformin thrice daily; this group is hereafter referred to as the MET group.
Treatment response was assessed by histological examination of the D&C specimens obtained after 12 weeks. The responses were classified into three categories. CR was defined as the reversion of EAH to proliferative or secretory endometrium. Partial response (PR) was defined as the regression of EAH to simple or complex hyperplasia without atypia. NR was defined as the persistence of EAH, and progressive disease (PD) was defined as the appearance of EC in EAH patients. After reconfirmation of normal liver and kidney function, use of oral contraceptives for 12 weeks was recommended in patients with CR. The same treatment was repeated for another 12 weeks in patients with PR. Patients with NR chose to either follow the same protocol for another 12 weeks or undergo an operation. Operation was recommended for PD patients. In this study, 12 weeks was set as an endpoint; therefore, data collected after the endpoint, which are included in another study, are not shown.
Nonparameter tests (two-sample Mann-Whitney test) were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). A value of p<0.05 was considered statistically significant. A comparison of response rates was performed between the MA and MET groups. In addition, we compared the efficacies of the two therapies in the presence or absence of MS in patients.
Thirty patients were enrolled initially, and 16 patients were included in the final analysis (Fig. 1). Of the 30 patients, 16 completed 12 weeks of therapy, and 14 were excluded, which consisted of eight patients who chose to undergo an operation and were remitted, three patients who were lost during follow-up, and three patients who had incomplete data (no blood test results available). Each group included eight patients. Table 1 lists the general information of the 16 patients. The mean age of the patients was 35.2±5.8 years (standard deviation, SD). The mean ages of the MA and MET group patients were 34±7.1 and 36.4±4.2 years, respectively. There was no statistically significant difference in age distribution between the two groups (p=0.433). All patients were married, and 37.5% of the patients (6/16) had a history of infertility. Furthermore, 50% of the patients (8/16) met the MS criteria, and 50% of the patients (4/8) had MS in both the MA and MET groups.
Of the 16 patients, 50% (8/16) had CR, 12.5% (2/16) had PR, and 37.5% (6/16) had NR. No patient displayed PD (Table 1). The response rate seemed better in the MET group than in the MA group, although the p-value was above 0.05 (p=0.105). Based on the response distribution, the MET group had a higher CR rate than did the MA group (75% vs. 25%). Moreover, the MET group had lower PR and NR rates than did the MA group (PR rates, 0% vs. 25%; NR rates, 25% vs. 50%, respectively) (Fig. 2). As shown in Table 2, ER and PgR expression in the endometrium in patients showed hardly any obvious differences before and after therapy. In addition, no severe side effects were observed during the 12 weeks of therapy. Only three patients receiving metformin experienced slight nausea, which was relieved without intervention and had no effect on metformin administration and subsequent therapy.
MS had no effect on metformin therapy in EAH patients with metabolic disorder in this study. On the basis of whether MS was present, we compared the treatment efficacies between the MET and MA groups (Fig. 3). Regardless of whether MS was present, the MET group showed the same CR rate of 75%, which was higher than that of the MA group. When MS was present, the CR rate of the MA group was 50%, and the p-value was 0.495 when the response condition was compared between the two groups. The CR rates of patients without MS were 75% and 0% in the MET and MA groups, respectively, and there was no significant difference in response distribution between the groups (p=0.127).
Through January 2014, no relapse occurred in the eight CR patients; one PR patient was lost, whereas another had CR after subsequent progesterone therapy and delivered a healthy child. Of the six NR patients, two achieved CR after follow-up treatment, and two showed no improvement and chose to undergo an operation; the other two patients were lost.
In our study, adjunctive metformin therapy outperformed MA monotherapy in the fertility-sparing treatment of EAH patients. The therapeutic trend is clear, although the p-values were above 0.05. Statistical power calculated by power analysis was 0.518, which suggests that the limited number of patients in this study may have contributed to the insignificant p-values. However, although this was just a pilot study with a limited number of patients, the adjunctive metformin therapy had a higher CR rate than did the MA monotherapy in EAH patients, which may suggest that adjunctive therapy is worthy of further study in a large number of patients.
Our study showed that after 12 weeks of therapy, the CR rate of the MET group was 75%, which was higher than that (25%) of the MA group, and the CR rate was similar to the resolution rate (approximately 70%) with different doses of MPA (500 to 1,000 mg/day or MA at 80 to 400 mg/day) reported in most studies, but the resolution time was shorter (3 months vs. 6 to 18 months) [1,6,7,17,18,19]. There are also reports of a higher CR rate with a long follow-up period in EAH treatment. One study reported an 85.6% regression rate in EAH patients, and the median follow-up time varied from 11 to 76.5 months [6]. Another study reported an 84.2% CR rate after a 5-year follow-up. Moreover, in a study by Ushijima et al. [5], all EAH patients achieved resolution with a fixed daily dose of 600 mg of MPA after 26 weeks. There are two factors that possibly contributed to the high CR rate in that study. One was that hysteroscopy was chosen for efficacy evaluation and assessment after treatment, which is better than D&C for examining and selecting endometrial lesions for pathological diagnosis. The other factor was that a high dose of MPA was used. However, adverse effects including weight gain, liver dysfunction, and abnormal blood coagulation were observed in that study [5]. Given the treatment periods and potential adverse effects, our study showed superior results of adjunctive metformin therapy with a 75% CR rate in only 3 months, which suggests that adjunctive metformin therapy might be a better choice than MA monotherapy for treating EAH patients. However, because our study has a limited number of patients, the efficacies of the therapies should be examined in a larger population.
We also found that the presence or absence of MS had no effect on the efficacy of adjunctive metformin treatment in EAH patients who met at least one MS criterion in our study. Both patients with and those without MS had a higher CR rate in the MET group than in the MA group. Because the number of patients in this study was limited, these effects need to be further verified in a study with a larger number of patients.
No irreversible side effects were detected during the entire course of our study, which further implies that adjunctive metformin is safe and potent for the treatment of EAH patients. As an oral antidiabetic drug, metformin is usually prescribed for type 2 diabetic patients. In recent years, it has been well demonstrated to be safe and beneficial for the treatment of nondiabetic patients with cancers [10,12], polycystic ovary syndrome [20], and other diseases [21].
In addition to its positive role in the amelioration of endometrial response to progestins in vivo, metformin has been demonstrated to have multiple effects. Metformin may affect endometrial pathology by upregulating PgR expression [22], downregulating glyoxalase I expression [23], modulating the mammalian target of rapamycin pathway, and blocking the epidermal growth factor signaling pathway to inhibit cell proliferation and improve progesterone therapy [24]. Clinically, metformin can improve MS and lower insulin and testosterone levels [10]. We believe that it may also work in EAH treatment; however, further research is needed.
Our study has some limitations, one being the limited sample size. More patients and a longer follow-up period are required. In addition, data on pregnancy outcomes, long-term side effects, and the association between insulin metabolism and therapeutic effects are still unclear and need to be examined in a larger population.
In conclusion, MET may be a potential alternative treatment for EAH patients. The presence or absence of MS may have no effect on the efficacy of adjunctive metformin therapy in EAH patients with metabolic disorders.
Figures and Tables
Fig. 1
Study design. CR, complete response; MA, megestrol acetate; MET, metformin plus MA; NR, no response; PR, partial response.

Fig. 2
Response distribution in the megestrol acetate (MA) and metformin plus MA (MET) groups in the study. Response distribution was compared between the two groups (p=0.105). CR, complete response; NR, no response; PR, partial response.

Fig. 3
Response distribution in the presence and absence of metabolic syndrome (MS) in the megestrol acetate (MA) and metformin plus MA (MET) groups. (A) It shows the response rates of the patients who did not meet the MS criteria in the two groups. Response distribution in the absence of MS was compared between the two groups (p=0.127). (B) It shows the response rates of the patients who met the MS criteria in the two groups. Response distribution in the presence of MS was compared between the two groups (p=0.495). CR, complete response; NR, no response; PR, partial response.
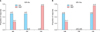
Table 2
Histological findings, estrogen receptor and progesterone receptor expression, and treatment response of the patients

CAH, complex hyperplasia with atypia; CH, complex hyperplasia without atypia; CR, complete response; MA, megestrol acetate; MET, metformin plus MA; NR, no response; PE, proliferative endometrium; PR, partial response; SE, secretory endometrium.
*Staining distribution (percentage positive cells): -, ≤5%; 1+, 6%-25%; 2+, 26%-50%; 3+, 51%-75%; and 4+, 76%-100% (all reactions exhibited moderate to strong staining intensity). †Primary pathologic diagnosis was done in other hospitals and confirmed by pathologists in our hospital, all of which the expression of hormone receptors were missed.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China, 2012 (NSFC No.: 81101953), Shanghai Municipal Science Foundation, 2013 (Project No.: 134119a4500), National Natural Science Foundation of China (NSFC No.: 81210108021), and Shanghai Municipal Science Foundation, 2011 (Project No.: 11ZR1404300).
References
1. Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012; 125:263–270.
2. Nucera G, Mandato VD, Gelli MC, Palomba S, La Sala GB. Gonadotropin releasing hormone agonist and levonorgestrel-intrauterine device followed by in vitro fertilization program as management strategy for an infertile endometrial cancer patient: a case report. Gynecol Endocrinol. 2013; 29:219–221.
3. Brown AJ, Westin SN, Broaddus RR, Schmeler K. Progestin intrauterine device in an adolescent with grade 2 endometrial cancer. Obstet Gynecol. 2012; 119(2 Pt 2):423–426.
4. Jasonni VM, Franceschetti F, Ciotti P, Bulletti C, Vignudelli A, Marabini A, et al. Treatment of endometrial hyperplasia with cyproterone acetate histological and hormonal aspects. Acta Obstet Gynecol Scand. 1986; 65:685–687.
5. Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007; 25:2798–2803.
6. Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012; 207:266.
7. Penner KR, Dorigo O, Aoyama C, Ostrzega N, Balzer BL, Rao J, et al. Predictors of resolution of complex atypical hyperplasia or grade 1 endometrial adenocarcinoma in premenopausal women treated with progestin therapy. Gynecol Oncol. 2012; 124:542–548.
8. Ricciardi E, Maniglio P, Frega A, Marci R, Caserta D, Moscarini M. Fertility-sparing treatment of endometrial cancer precursors among young women: a reproductive point of view. Eur Rev Med Pharmacol Sci. 2012; 16:1934–1937.
9. Shen ZQ, Zhu HT, Lin JF. Reverse of progestin-resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstet Gynecol. 2008; 112(2 Pt 2):465–467.
10. Campagnoli C, Abba C, Ambroggio S, Brucato T, Pasanisi P. Life-style and metformin for the prevention of endometrial pathology in postmenopausal women. Gynecol Endocrinol. 2013; 29:119–124.
11. Session DR, Kalli KR, Tummon IS, Damario MA, Dumesic DA. Treatment of atypical endometrial hyperplasia with an insulin-sensitizing agent. Gynecol Endocrinol. 2003; 17:405–407.
12. Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013; 37:207–218.
13. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645.
14. Chen CH, Huang MC, Kao CF, Lin SK, Kuo PH, Chiu CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013; 74:e424–e430.
15. Kurman RJ, Ellenson LH, Ronnett BM. Blaustein's pathology of female genital tract. 6th ed. New York: Springer;2011.
16. Vang R, Gown AM, Barry TS, Wheeler DT, Ronnett BM. Immunohistochemistry for estrogen and progesterone receptors in the distinction of primary and metastatic mucinous tumors in the ovary: an analysis of 124 cases. Mod Pathol. 2006; 19:97–105.
17. Mentrikoski MJ, Shah AA, Hanley KZ, Atkins KA. Assessing endometrial hyperplasia and carcinoma treated with progestin therapy. Am J Clin Pathol. 2012; 138:524–534.
18. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012; 125:477–482.
19. Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010; 203:547.
20. Ganie MA, Khurana ML, Nisar S, Shah PA, Shah ZA, Kulshrestha B, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013; 98:3599–3607.
21. Lexis CP, van der Horst IC, Lipsic E, van der Harst P, van der Horst-Schrivers AN, Wolffenbuttel BH, et al. Metformin in non-diabetic patients presenting with ST elevation myocardial infarction: rationale and design of the glycometabolic intervention as adjunct to primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial. Cardiovasc Drugs Ther. 2012; 26:417–426.
22. Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011; 126:113–120.
23. Zhang Z, Dong L, Sui L, Yang Y, Liu X, Yu Y, et al. Metformin reverses progestin resistance in endometrial cancer cells by downregulating GloI expression. Int J Gynecol Cancer. 2011; 21:213–221.
24. Hanna RK, Zhou C, Malloy KM, Sun L, Zhong Y, Gehrig PA, et al. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol. 2012; 125:458–469.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download