Abstract
Objective
This study was conducted to ascertain the correlation between preserved pelvic nerve networks and bladder function after laparoscopic nerve-sparing radical hysterectomy.
Methods
Between 2009 and 2011, 53 patients underwent total laparoscopic radical hysterectomies. They were categorized into groups A, B, and C based on the status of preserved pelvic nerve networks: complete preservation of the pelvic nerve plexus (group A, 27 cases); partial preservation (group B, 13 cases); and complete sacrifice (group C, 13 cases). To evaluate bladder function, urodynamic studies were conducted preoperatively and postoperatively at 1, 3, 6, and 12 months after surgery.
Results
No significant difference in sensory function was found between groups A and B. However, the sensory function of group C was significantly lower than that of the other groups. Group A had significantly better motor function than groups B and C. No significant difference in motor function was found between groups B and C. Results showed that the sensory nerve is distributed predominantly at the dorsal half of the pelvic nerve networks, but the motor nerve is predominantly distributed at the ventral half.
Total laparoscopic radical hysterectomies are technically feasible for not only early stage but also advanced stage cervical carcinomas [1,2,3,4]. However, bladder dysfunction after radical hysterectomies has been well documented. Damage to the pelvic nerve plexus (inferior hypogastric plexus) and its vesical branches-the pathways for neurogenic bladder control [5,6,7]-is the main cause of dysfunction.
Total laparoscopic nerve-sparing radical hysterectomy has been developed recently for early stage cervical carcinoma, with a technical procedure already established. In this procedure, the hypogastric nerves, instead of the pelvic nerve plexus and its vesical branches, are preserved at several steps during a classical radical hysterectomy [8,9]. Consequently, the hypogastric nerves constitute the main anatomical landmark of nerve-sparing radical hysterectomy because they form the upper limit of the pelvic nerve plexus and the vesical branches.
However, the origin and distribution of the pelvic nerve plexus have not been fully described. If anatomical details of the pelvic nerve plexus and the vesical branches could be elucidated, various types of nerve-sparing laparoscopic radical hysterectomies would be achievable, with procedures adaptable to the level of cervical carcinoma risk.
Many reports assess bladder function after nerve-sparing radical hysterectomy by measuring the residual volume of urine after voiding [10]. Benedetti-Panici et al. [11] described that only 15% of patients who underwent radical hysterectomies had a residual urine volume >30% of their bladder capacity. However, 76% of patients developed bladder dysfunction during the 12 months after surgery [11]. Therefore, measuring the residual volume of urine after voiding might not be sufficient to evaluate bladder function. Urodynamic evaluation is necessary to ascertain bladder dysfunction after nerve-sparing radical hysterectomies.
For this study, we used fresh cadavers to investigate the detailed anatomy of the pelvic nerve plexus and its vesical branches to define three types of nerve-sparing laparoscopic radical hysterectomies. We evaluated bladder function after these procedures using urodynamic studies.
We conducted this study to reveal the correlation between preserved pelvic nerve networks and bladder function after laparoscopic nerve-sparing radical hysterectomy.
This study included 53 patients who underwent total laparoscopic radical hysterectomies between April 2009 and April 2011 because of cervical carcinomas. Table 1 shows the clinical backgrounds of these 53 patients, who were categorized into groups A, B, and C based on the status of the pelvic nerve systems, as described below.
(1) Group A: Patients whose hypogastric nerves were preserved completely (pelvic nerve plexus was preserved completely) on both sides (conventional nerve-sparing technique).
(2) Group B: Patients whose hypogastric nerves were sacrificed and pelvic splanchnic nerves were partially preserved (pelvic nerve plexus was partially preserved) on both sides (radical nerve-sparing technique).
(3) Group C: Patients whose hypogastric and pelvic splanchnic nerves were completely sacrificed (pelvic nerve plexus was completely sacrificed) on both sides (non-nerve-sparing technique).
The study excluded patients with mixed pelvic nerve system-sparing status (e.g., the right side is categorized into group A, and the left side is categorized into group C).
To prevent thermal injury to preserved nerve systems, we used an ultrasonic dissection device (Harmonic ACE, Ethicon Endo-Surgery, Tokyo, Japan) or a vessel sealing device (LigaSure, Covidien, Tokyo, Japan) around the pelvic nerve network. To evaluate bladder function, urodynamic studies were conducted before surgery and at 1, 3, 6, and 12 months after surgery. No patient took medication for neurogenic bladder dysfunction (for example, a cholinergic agent or α1 blocker) during the follow-up period.
The indications for the procedures in groups A, B, and C are defined below. The conventional nerve-sparing procedure (group A) was applied to patients with a tumor <3 cm in size, when the histology was not indicative of a neuroendocrine type and lymph node swelling and parametrial invasion were not suspected before surgery. The radical nerve-sparing procedure (group B) was applied to patients with a tumor 3 to 4 cm in size, including a neuroendocrine type, and with no obvious evidence of lymph node metastasis or parametrial invasion. The non-nerve-sparing procedure (group C) was applied to other high-risk cervical carcinomas. The study excluded patients who received adjuvant radiation therapy because radiation therapy might worsen bladder function. Informed consent was obtained from all patients before surgery.
Several reports have described procedures for total laparoscopic nerve-sparing radical hysterectomy [8,9]. However, all of these procedures are categorized into the conventional nerve-sparing group (group A) as described above. No radical nerve-sparing procedure (group B) has ever been described.
The pelvic nerve plexus consists of hypogastric nerves and pelvic splanchnic nerves, as well as sacral splanchnic nerves. The hypogastric nerves join the pelvic nerve plexus from the superior ventral side. The pelvic splanchnic nerves, which originated from the ventral rami S2 to S4, join from the lateral dorsal side [12]. To accomplish the conventional nerve-sparing procedure (group A), the parametrium and paracolpium tissue should be transected just above the hypogastric nerves, allowing the pelvic nerve plexus to be preserved. Consequently, in the group A procedure, exposure of the pelvic splanchnic nerves is not necessary. In contrast, during the radical nerve-sparing procedure (group B), the hypogastric nerves are sacrificed and the pelvic nerve plexus is partially preserved to attain more radicality than with the conventional nerve-sparing procedure (group A). Therefore, the denudation of pelvic splanchnic nerves is necessary.
Previous reports describing total laparoscopic nerve-sparing radical hysterectomy have not explained laparoscopic techniques to elucidate the pelvic splanchnic nerves. We describe our innovative techniques to elucidate pelvic nerve networks (hypogastric nerves, pelvic splanchnic nerves, pelvic nerve plexus, and its vesical branches), based on a cadaveric study.
Our cadaveric study specifically examined the exposed complete anatomical structure of the pelvic nerve networks. Through this study, we established our original techniques to expose these networks. The rectum was resected beforehand to widely develop pararectal and presacral spaces. Then the sacral nerves (S1 to S4) were exposed at the anterior sacral foramina. This site is the origin of the pelvic splanchnic nerves, which join the pelvic nerve plexus. Consequently, this identification engenders the complete exposure of the pelvic splanchnic nerves. After identifying the hypogastric nerves and pelvic splanchnic nerves, the pelvic nerve plexus and its vesical branches can easily be exposed. The cadaveric study elucidated that the pelvic nerve plexus is approximately 3 cm wide (Fig. 1). After developing the pararectal space widely, the hypogastric nerves can be observed easily along the lateral sides of the mesorectum. The lymph node tissue around the internal iliac region is removed completely, and then the S2 to S3 roots are identifiable beneath the fascia of the piriform muscle. The pelvic splanchnic nerves, which join the pelvic nerve plexus, also are visible as visceral branches originating from S2 to S3 roots after lymph node tissue around the cardinal ligament is meticulously removed (Fig. 2). After transecting the vessel part of the cardinal ligament, the pelvic nerve plexus can be exposed. When adipose tissue around the posterior part of the vesicouterine ligament is removed completely, the vesical branches from the pelvic nerve plexus can be exposed as fibrous tissue connecting the pelvic nerve plexus with the bladder.
At the point where we transect the uterosacral ligament and paracolpium tissue, we are able to control the radicality of the procedure. The uterosacral ligament and the paracolpium tissue are transected above the hypogastric nerves for group A type radicality, between the hypogastric nerves and pelvic splanchnic nerves for group B, or below the pelvic splanchnic nerves for group C (Fig. 3).
We conducted urodynamic studies with Ellips (Sword Medical, Dublin, Ireland) five times: before surgery, and at 1, 3, 6, and 12 months after surgery.
The evaluation of sensory and motor function of the bladder in this study required two parameters for urodynamic analysis: first desire to void (FDV) and PdetQmax. FDV, the capacity of the bladder when the patient feels the first sensation to void, is used to evaluate sensory function. PdetQmax, the detrusor contraction pressure at maximum flow, is used to evaluate motor function.
The difficulty with the current form of analysis is that the bladder function of some patients is already weak or damaged preoperatively, making it difficult to accurately assess postoperative bladder function. Therefore, we have developed an original function ratio to evaluate bladder function.
Function ratio (FDV)=FDV preoperative/FDV postoperative.
Function ratio (PdetQmax)=PdetQmax postoperative/PdetQmax preoperative.
PdetQmax is expected to be in proportion to bladder function, and FDV is expected to be in inverse proportion to bladder function. Therefore, the function ratio formulas (FDV, PdetQmax) differ.
We performed 53 total laparoscopic radical hysterectomies without complications. Based on the preservation of pelvic nerve system status, we categorized them into group A (n=27), group B (n=13), and group C (n=13).
Fig. 4 shows the function ratio (FDV and PdetQmax) data for respective groups at 1, 3, 6, and 12 months after surgery. The recovery of sensory function in groups A and B was visible within 12 months after surgery, and sensory function at 6 and 12 months was statistically higher than that at 1 month after surgery.
As with sensory function, the recovery of motor function in group A was visible within 12 months after surgery, and motor function at 6 and 12 months was statistically higher than that at 1 month after surgery. In contrast, in group B, significant recovery of motor function was not visible within 12 months after surgery. In group C, recovery of sensory and motor function was not at all visible. All the function ratio data of sensory and motor functions in group C were zero.
Fig. 5 shows the function ratio (FDV and PdetQmax) data of respective groups at 12 months after surgery. For sensory function, the function ratio data of groups A and B were significantly higher than those of group C. We found no significant difference between groups A and B. The motor function ratio data of group A was significantly higher than that in groups B and C. However, no significant difference was found between groups B and C.
Total laparoscopic radical hysterectomy for cervical carcinoma is an accepted modality not only because of its technical feasibility but also because of its oncologic outcomes [13,14,15,16,17,18]. However, bladder dysfunction after laparoscopic radical hysterectomy is liable to decrease the quality of life owing to the physical and mental stress that accompanies postoperative disorders. Nerve-sparing techniques have been established to maintain postoperative quality of life [8,9].
Several previous papers described that the careful identification and preservation of the hypogastric (sympathetic) nerves is crucial to minimize bladder function impairment. Therefore, the hypogastric nerves are regarded as the anatomical landmark to accomplish total laparoscopic nerve-sparing radical hysterectomy.
Bladder function following conventional total laparoscopic nerve-sparing radical hysterectomy is quite good. However, in some cases the radicality of this procedure is insufficient. Therefore, its applicability is expected to be limited to early stage cervical carcinoma [19]. Greater radicality is necessary for intermediate stage or advanced stage cervical carcinoma. However, nerve-sparing techniques have never been applied in such cases. In some intermediate-risk cervical carcinoma cases, non-nerve-sparing radical hysterectomy tends to be overtreatment. We suggest applying another type of nerve-sparing technique for these cases.
The pelvic nerve plexus appears as a mesh, and consists mainly of hypogastric nerves and pelvic splanchnic nerves. The hypogastric nerves are located at the ventral edge, and the pelvic splanchnic nerves are at the dorsal edge of the pelvic nerve plexus [12]. Therefore, the pelvic nerve plexus has some width anatomically. A nerve-sparing technique that is more radical than conventional nerve-sparing radical hysterectomy is possible. This innovative radical technique requires the complete exposure of the pelvic nerve plexus. Many earlier reports describe detailed investigations of the pelvic anatomy with formalin-fixed cadavers. However, we were unable to apply this knowledge to practical surgery because the anatomy in formalin-fixed cadavers differs from the practical anatomy encountered in the surgical field. To investigate practical pelvic anatomy, we performed a fresh cadaveric study with a laparoscope. This procedure was performed in the same way as a practical surgery. Consequently, we were able to establish our original procedure, which we have called radical nerve-sparing technique.
To accurately assess postoperative bladder function, we decided not to rely solely on the residual volume of the bladder after voiding. Instead, we applied urodynamic analysis. Several studies have conducted urodynamic analyses after radical hysterectomy, but no consensus on these procedures has been reached, perhaps because approximately 80% of cervical carcinoma patients already have some degree of bladder dysfunction before surgery. It is therefore extremely difficult to evaluate the effect of nerve-sparing techniques [20]. In this study, we define our original index (function ratio) to control for a scattering of data of urodynamic studies before surgeries.
Urodynamic studies have many parameters. The PdetQmax is the standard parameter for evaluating bladder motor function. However, the urodynamic study has three parameters regarding bladder sensory function: first sensation of filling, FDV, and strong desire to void. The best parameter for the evaluation of bladder sensory function has not been defined.
The first sensation of bladder filling is weak and inconsistent, and is possibly dependent on cortical fluctuation. Impulses related to the FDV traverse through the pelvic nerves, and impulses for the sensation of a full bladder (strong desire to void) traverse through the pudendal nerves [21]. Therefore, we chose the FDV as the most effective parameter to evaluate bladder sensory function after the nerve-sparing procedure.
Ralph et al. [22] reported that bladder function after radical hysterectomy can be improved within 12 months after surgery. Our results also showed that the preserved nerve function (the sensory function of groups A and B, and the motor function of group A) can be improved within 12 months after surgery. Therefore, we analyzed the data at 12 months after surgery to ascertain the correlation between the preserved pelvic nerve networks and bladder function after laparoscopic nerve-sparing radical hysterectomy.
Based on results of this study, we can suggest that the distributions of sensory nerves and motor nerves differ. The sensory nerve is distributed predominantly at the lower (dorsal) half of the pelvic nerve networks. Therefore, the sensory functions of groups A and B are statistically equivalent, and the sensory function of group C is significantly lower than that of groups A and B. In contrast, the motor nerve is distributed predominantly at the upper (ventral) half of the pelvic nerve networks. Therefore, the motor function of group A is significantly more preserved compared to that of groups B and C. The motor functions of groups B and C are damaged similarly; they show no mutually significant difference.
This is the first paper to describe bladder dysfunction after total laparoscopic radical hysterectomy with the point of sensory and motor nerve functions selectively, and to reveal that the distributions of motor and sensory nerves of the bladder differ.
In conclusion, results of this study show that the various types of total laparoscopic nerve-sparing radical hysterectomies are technically feasible, and that they can be tailored to the risk of cervical cancer. To determine the applicability of these procedures, it is necessary to follow-up patients and to evaluate survival data.
Figures and Tables
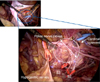 | Fig. 1Detailed pelvic nerve networks around right parametrium in a fresh cadaver. The pelvic nerve plexus is approximately 3 cm wide, and consists of a hypogastric nerve and pelvic splanchnic nerves. |
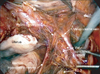 | Fig. 2The pelvic nerve networks of the right side before transecting the cardinal ligament. At this point, the vesical branch of the pelvic nerve plexus is not elucidated. |
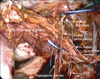 | Fig. 3After transecting the cardinal ligament, the complete structure of the pelvic nerve networks, including the vesical branch, can be elucidated. This figure shows the cutting line of groups A, B, and C. |
 | Fig. 4Function ratio of first desire to void (FDV) and the detrusor contraction pressure at maximum (PdetQmax) at 1, 3, 6, and 12 months after surgery. (A) Group A. (B) Group B. (C) Group C. The standard error of the mean of the function ratio data. p-values are derived from two-tailed tests. The asterisk means not significant. *p≥0.05. |
 | Fig. 5Function ratio of first desire to void (FDV) and the detrusor contraction pressure at maximum (PdetQmax) of groups A, B, and C at 12 months after surgery. The standard error of the mean of the function ratio data. p-values are derived from two-tailed tests. The asterisk means not significant. *p≥0.05. |
Table 1
Clinical background of the patients

Ad, adenocarcinoma; Adsq, adenosquamous cell carcinoma; AWD, alive with disease; CBDCA, carboplatin; CDGP, nedaplatin; CPT-11, irinotecan; DC, docetaxel and carboplatin; DFI, disease free interval; DOD, death of disease; LN, lymph node; NED, no evidence of disease; SCC, squamous cell carcinoma; Small, small cell carcinoma; TC, taxol and carboplatin; 5FU, fluorouracil.
Notes
Supplementary material for this article can be found at www.ejgo.org.
A video clip is available at http://ejgo.org/.
References
1. Simsek T, Ozekinci M, Saruhan Z, Sever B, Pestereli E. Laparoscopic surgery compared to traditional abdominal surgery in the management of early stage cervical cancer. Eur J Gynaecol Oncol. 2012; 33:395–398.
2. Pellegrino A, Vizza E, Fruscio R, Villa A, Corrado G, Villa M, et al. Total laparoscopic radical hysterectomy and pelvic lymphadenectomy in patients with Ib1 stage cervical cancer: analysis of surgical and oncological outcome. Eur J Surg Oncol. 2009; 35:98–103.
3. Vizza E, Pellegrino A, Milani R, Fruscio R, Baiocco E, Cognetti F, et al. Total laparoscopic radical hysterectomy and pelvic lymphadenectomy in locally advanced stage IB2-IIB cervical cancer patients after neoadjuvant chemotherapy. Eur J Surg Oncol. 2011; 37:364–369.
4. Colombo PE, Bertrand MM, Gutowski M, Mourregot A, Fabbro M, Saint-Aubert B, et al. Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: surgical morbidity and oncological results. Gynecol Oncol. 2009; 114:404–409.
5. Glahn BE. The neurogenic factor in vesical dysfunction following radical hysterectomy for carcinoma of the cervix. Scand J Urol Nephrol. 1970; 4:107–116.
6. Seski JC, Diokno AC. Bladder dysfunction after radical abdominal hysterectomy. Am J Obstet Gynecol. 1977; 128:643–651.
7. Fishman IJ, Shabsigh R, Kaplan AL. Lower urinary tract dysfunction after radical hysterectomy for carcinoma of cervix. Urology. 1986; 28:462–468.
8. Kavallaris A, Hornemann A, Chalvatzas N, Luedders D, Diedrich K, Bohlmann MK. Laparoscopic nerve-sparing radical hysterectomy: description of the technique and patients' outcome. Gynecol Oncol. 2010; 119:198–201.
9. Liang Z, Chen Y, Xu H, Li Y, Wang D. Laparoscopic nerve-sparing radical hysterectomy with fascia space dissection technique for cervical cancer: description of technique and outcomes. Gynecol Oncol. 2010; 119:202–207.
10. Plotti F, Zullo MA, Montera R, Angioli R, Pierluigi BP. Bladder function after laparoscopic nerve-sparing radical hysterectomy. Gynecol Oncol. 2011; 120:315.
11. Benedetti-Panici P, Zullo MA, Plotti F, Manci N, Muzii L, Angioli R. Long-term bladder function in patients with locally advanced cervical carcinoma treated with neoadjuvant chemotherapy and type 3-4 radical hysterectomy. Cancer. 2004; 100:2110–2117.
12. Yamaguchi K, Kobayashi M, Kato T, Akita K. Origins and distribution of nerves to the female urinary bladder: new anatomical findings in the sex differences. Clin Anat. 2011; 24:880–885.
13. Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009; 16:1316–1323.
14. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical cancer. Gynecol Oncol. 2007; 105:176–180.
15. Taylor SE, McBee WC Jr, Richard SD, Edwards RP. Radical hysterectomy for early stage cervical cancer: laparoscopy versus laparotomy. JSLS. 2011; 15:213–217.
16. Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011; 156:83–86.
17. Sobiczewski P, Bidzinski M, Derlatka P, Panek G, Danska-Bidzinska A, Gmyrek L, et al. Early cervical cancer managed by laparoscopy and conventional surgery: comparison of treatment results. Int J Gynecol Cancer. 2009; 19:1390–1395.
18. Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012; 23:903–911.
19. Park NY, Chong GO, Hong DG, Cho YL, Park IS, Lee YS. Oncologic results and surgical morbidity of laparoscopic nerve-sparing radical hysterectomy in the treatment of FIGO stage IB cervical cancer: long-term follow-up. Int J Gynecol Cancer. 2011; 21:355–362.
20. Lin HH, Yu HJ, Sheu BC, Huang SC. Importance of urodynamic study before radical hysterectomy for cervical cancer. Gynecol Oncol. 2001; 81:270–272.
21. Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002; 59:5 Suppl 1. 43–50.
22. Ralph G, Tamussino K, Lichtenegger W. Urological complications after radical abdominal hysterectomy for cervical cancer. Baillieres Clin Obstet Gynaecol. 1988; 2:943–952.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download