Abstract
We have designed a five-year multicentre prospective cohort study in women who are both human papillomavirus (HPV)-positive with either atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) of cervix. This study aimed to analyze the risk of developing a high-grade squamous intraepithelial lesion (HSIL) from either ASCUS or LSIL in HPV-positive women, so called 'progression' rate, to investigate differences in the progression rates according to HPV type-specific infection, and to evaluate the various factors associated with the persistence or clearance of HPV infection in the Korean population. At present, the study protocol composed of cervical cytology, HPV DNA testing, and questionnaire have been conducted actively since the first participant was enrolled in 2010. This study is the first nationwide Korea HPV cohort study. Our data will provide valuable information about not only the ambiguous cytology results of ASCUS and LSIL but also the effect of the specific HPV type and other various factors on the progression to HSIL. Finally, the results of our study will be helpful and applicable to determine the primary cervical cancer prevention strategies.
Cervical cancer is the second most common malignancy and a leading cause of cancer-related death in women worldwide. Although the incidence and mortality from cervical cancer have markedly decreased due to high-quality screening with cytology and the development of cervical vaccines, the disease burden remains to be significant with 530,000 new cases and 275,000 deaths from cervical cancer in 2008 [1]. In the United States, the estimated number of new cases of cervical cancer expected to occur in 2012 have been reported to be 12,170 with estimation of 4,220 deaths [2]. In 2009, there were 3,733 cases that were newly diagnosed, ranking cervical cancer the sixth most common cancer among women in Korea [3].
The human papillomavirus (HPV) is the most important risk factor for carcinogenesis of cervical cancer. It is broadly accepted that persistent infection by HPV genotype plays an important role in the development of cervical premalignant and malignant epithelial lesions. Other demographic, genetic, immunologic and environmental factors can also affect the persistence of the HPV infection and further carcinogenesis of the cervical cancer [4]. Recent large observational studies have demonstrated that the distribution of HPV genotypes is heterogeneous among women from different populations [5]. Therefore, the acquisition of population-based data is crucial to the development of new screening and management protocols for cervical neoplasms as well as to the assessment of the effect of future vaccination on HPV infections.
Although many previous reports have addressed the considerable risk of a cervical intraepithelial neoplasia (CIN) of grade 2 or greater in women with the high-grade squamous intraepithelial lesion (HSIL) cytology [6-8] and virtually all physicians agree that HSIL requires colposcopic examination or immediate excisional biopsy, the clinical significance of atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) Pap smear results remains vague. Eventually, the progression rate from CIN1 to CIN2 or CIN3 is linked to the conservative clinical management of ASCUS and LSIL. Patients with ASCUS or LSIL exhibit a wide spectrum of histologic results, with most showing mild abnormalities of less than CIN2 [9] with spontaneous regression, however invasive carcinoma is also diagnosed in rare cases. However, few studies are available to approximate the rate of 'progression' from either ASCUS or LSIL to HSIL and to indicate the factors associated with progression. In the Korea HPV cohort study, we therefore aimed to calculate the progression rate from ASCUS or LSIL to HSIL in HPV-positive women and to investigate if the progression would be influenced by the specific types of HPV infection or specific traits of Korean population, which we categorized into epidemiological, virological, environmental and genetic factors.
In 2010, women presenting for routine cervical cancer screening was initiated to be enrolled in this prospective multicenter cohort study from five university medical centers nationwide, funded by the Korea Centers for Disease Control and Prevention. The institutions are distributed in four of seven metropolitan cities in Korea: Cheil General Hospital and Women's Healthcare Center of the Kwandong University, Seoul St. Mary's Hospital of the Catholic University of Korea, Chonnam University Hospital, Dongsan Hospital of the Keimyung University, and Busan Paik Hospital of the Inje University. From these five investigators, we constructed an academic committee composed of a research group and a council team; the former is subcategorized into clinical investigation responsible for biological samples, virus research, epidemiological, genetic, pathological, and biological research, while the latter is responsible for medical ethics and preventive medicine. The Korea cohort study is an organized and systematic research project with a long-term schedule of follow-up to establish the infrastructure for HPV infection.
Patients eligible for inclusion are those aged from 20 to 60 years with an intact uterus who are HPV-positive with either ASCUS or LSIL cytology with informed consent. Exclusion criteria are pregnancy at initial evaluation, malignant disease including cervical cancer diagnosed at enrollment, psychological disease currently under treatment, hysterectomy for any reason at any phase of the study, and history of CIN treated within 6 months of enrollment. The sample size of 2,000 patients for five years of follow-up was determined using a valid method for calculation, as shown later.
Initial evaluation at enrollment consists of a physical examination, blood sampling and a questionnaire. The physical examination is comprised of a blood pressure, body weight and Initial evaluation at enrollment consists of a physical examination, blood sampling and a questionnaire. The physical examination is comprised of a blood pressure, body weight and height measurements, and a pelvic examination. For follow-up, women are examined by repeat cytology and HPV testing every 6 months and repeated the questionnaire and blood sampling every year. If the women do not visit the hospital beyond 12 months from the scheduled date or have hysterectomy for any other reasons, they will be considered to have met the requirements for dropping out of the study. A description of the salient parts of the procedure is presented in Table 1.
In case of the mild lesions of less than HSIL as seen by cytology during follow-up periods, it will be acceptable for clinicians to recheck a Pap smear and HPV test at 6 months later. However, if the cytology reveals HSIL or more troublesome results, colposcopy and biopsy will be necessary to provide histologic confirmation. When the histologic result reveals HSIL or worse (≥CIN2), the women will be treated by conization because HSIL is a target to calculate the progression rate as a primary endpoint. If there is a difference between the cytology results and biopsy histology, the latter should be considered as a more confirmative diagnosis. Nevertheless, if women present with HSIL or worse cytology, but only mild abnormalities on colposcopy, such as CIN1 or condyloma, they will be continued a regular follow-up. Fig. 1 depicts the main protocol regarding HPV testing and cervical cytology in the present study.
In this protocol, specific types of HPV infection are not independent factors that determine whether to perform a colposcopy or not. Compared with the previous results of the HPV test, the next follow-up would yield four kinds of change in HPV infection type: positive for the same type, positive for both the same type and additional types (new types of HPV), positive only for additional types, or negative for HPV, as shown in Fig. 1. Using these findings, we will evaluate the rates of persistence, clearance, and incidence of infection according to the specific type of HPV.
Residual biological samples (cervix DNA, plasma, serum, and buffy coat) collected and separated after liquid-based Pap smear and blood testing are delivered to Korea Biobank Network at Korea Centers for Disease Control and Prevention and stored at nitrogen tank. These specimens will then be available for future molecular investigations.
Persistent infection is defined as a specific type of HPV infection present in two consecutive HPV tests at a 6 months interval, and type-specific HPV clearance is defined as a negative result for a specific type of HPV following a previously positive result. Multiple infections are defined as being positive for two or more types of HPV in any phase of the study. Incident type-specific infection is defined as a positive result for a specific type of HPV for which the previous test was negative, and type-specific HPV reinfection is defined as a positive result for a specific type of HPV that has previously been cleared. On the bases of these definitions, the relationship between the status of HPV infection and the cytology result will be evaluated.
Demographic information of participants such as name and date of birth will be collected and included in the study database. For a thorough control of the subjects and samples, a unique study identification (ID) number and barcode will automatically be generated by the database and attached to all sample containers. To minimize variation in interpretation and handling of specimens, all materials including cytology, HPV test, and immunhistochemical study are to be transferred to a single institution (Cheil General Hospital and Women's Healthcare Center) and interpreted by one or more of a group of expert pathologists and medical laboratory technologists.
Initially, the results of the Pap smear and HPV testing obtained from participant hospitals in the study can be used if they are yielded within four weeks from enrollment. For cervical cytology, both conventional and liquid-based Pap smears are acceptable at baseline evaluation, but only the liquid-based Pap smears with the Cervex-Brush (Rovers Medical Devices, Oss, Netherlands) should be used during the followup examination. Using the cervical cells obtained from liquid-based cytology, immunostaining for the p16 and Ki-67 biomarker and HPV test may be performed. HPV detection will be conducted by a DNA microarray technique (DNA chip), which is based on the PCR method. Briefly, after amplification, PCR products are analyzed by electrophoresis and hybridized with radiolabeled generic probes for HPV. We consider types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, and 70 as having high oncogenic risk, and types 6, 7, 10, 11, 27, 32, 40, 42, 43, 44, 54, 55, 57, 61, 62, 72, 73, 81, 83, 84, and 91 as having low or non-oncogenic risk.
Colposcopic examination will be carefully conducted in the routine manner using green filter and saline application to remove cervical mucus. The entire sample for colposcopy should be inspected with not only 3% acetic acid but also iodine, and the sample should be graded according to the Reid colposcopic index, which is the most recently developed colposcopic grading system for the assessment of pre-invasive disease [10]. Then, colposcopic-directed biopsy is to be carried out in one to four of the most suspicious lesions.
Blood tests are to be performed for all participants enrolled in study. Each participant will provide 10 mL of blood, which will be centrifuged and stored at -70℃. Cell-mediated immunity and humoral immunity will be examined and the target biological markers for analysis are as follows: expression of human leukocyte antigen (HLA) class I and II alleles, HLA variant and polymorphism, interferon (INF)-γ, interleukin-10, tumor necrosis factor-α, transporter associated with antigen-processing protein-1, CD86, CD80, interferon regulatory factor-1, loss of chromosome 3p, 11p, and 13q, phosphatase and tensin homolog hypermethylation, p53 codon 72 polymorphism, p16 (INK4A) promoter hypermethylation, cell adhesion molecule 1 and T-lymphocyte maturation associated protein promoter methylation, expression of matrix metalloproteinase, cancer-relevant microRNAs, activation of NF-kappaB response gene, expression condition of dendritic cell, regulatory T cell, and lymphocyte.
We have developed a health questionnaire that is composed of the following categories: 1) sociodemographic status, 2) health-related lifestyle, 3) women-specific conditions, and 4) sexual habits that are administered by participants in person; and 1) disease history, 2) medication history, and 3) family history that are recorded by a trained investigator with a one-to-one interview. Specific details of the items are presented in Table 2. In the self-administered health questionnaires, if an answer is inadequate, an appointed investigator will confirm the information by asking the participants.
The Kelsey Method for cohort [11] was used to compute the minimum sample size by using the following inputs: a significance level of 5% and a statistical power of 80%. Based on the ASCUS-LSIL Triage Study (ALTS), the risk of CIN3 was 2.7% in women with noncarcinogenic HPV positivity for two years and 7.9% in carcinogenic HPV positivity [12]. In our gynecological clinical experience, 23% of women were assumed to be lost to follow-up. In addition, considering 40.4% of the incidence of 1 year-persistent HPV positivity in Korea [12], at least 1,881 women ended up being expected to be registered, thus, we aimed to obtain 2,000 participants for the study.
All analyses will be conducted using SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA). The baseline variables of study population will be presented as means±SD for continuous variables or frequencies (%) for categorical variables. General and clinical characteristics of subjects will be compared by using Pearson chi-square test or ANOVA. Multiple logistic regression models will be used to estimates odds ratios and 95% CI to quantify the associations between HPV infection and epidemiological, clinical and immunohistological characteristics after adjusting for confounding factors. Specifically, the association between the progression rate from ASCUS or LSIL to HSIL and a specific type of HPV as well as other characteristics, such as p16 and Ki-67 biomarker, sociodemographic status, obstetric history, sexual habits and so on will be investigated. Changes in persistence of HPV positivity and biochemical measurements during the follow-up period will be evaluated with a trend test. Also, Cox regression will be used to model the association of HPV infection with various factors. Hazard ratios and 95% CI will be estimated. A p<0.05 will be considered statistically significant.
The study is conducted according to the Korean law relating to the protection of patients participating in biomedical research. All of the research aims, study design, and the specific methods in the present study have been approved by the Institutional Review Board from all five of the participating hospitals and all participants will be asked for their written informed consents after having received written and oral information about the study. Throughout the research period, any minor change and new introduction in the aim and method will be embedded in the study only after approval of the ethical committee.
The Korea HPV cohort study is a research study targeting HPV-positive women with ASCUS or LSIL cytology. The study intends to determine the progression rate to HSIL (≥CIN2) and the associated factors in the Korean population. This will be a more comprehensive study than previous ones, since more subjects and more variables will be included compared to the existing research, and a multidisciplinary approach that will progress simultaneously will be employed for the first time in the Korea HPV cohort study.
The risk of subsequent HSIL following an ASCUS or LSIL has been reported to various extents in previous literature. Pretorius et al. [13] documented that among 2,490 women whose colposcopic diagnosis was CIN1 or less, the subsequent risk of CIN3 or cancer was found to be only 1.9%. Similarly, a recent report by Chen et al. [14] indicated less than 3% HSIL following an LSIL biopsy. On the other hand, in a review of literature, Ostor [15] stated that the likelihood of progression from CIN1 to CIN3 and invasive cancer was as high as 10% and 1%, respectively. Using 2,090 women with LSIL or HPV DNA-positive ASCUS cytology participating in the ALTS trial, Cox et al. [9] demonstrated that the cumulative risk of developing CIN2 or worse was 13% in cases of CIN1 confirmed with colposcopically directed biopsy during a two year follow-up.
Given this progression rate and management for CIN, attention has been focused on which CIN1 cases would be most likely to progress to CIN2 or worse. Among the numerous risk factors, HPV infection must be the most powerful element in the progression. From the subjects with histologic grade Given this progression rate and management for CIN, attention has been focused on which CIN1 cases would be most likely to progress to CIN2 or worse. Among the numerous risk factors, HPV infection must be the most powerful element in the progression. From the subjects with histologic grade <CIN2 at the first colposcopic examination in the ALTS trial, Walker et al. [16] reported that women with HPV-negative tests were at low risk for CIN2 regardless of the results of other tests such as Pap cytology and colposcopy, but the risk for an HPV-positive result overall was 19.2%. By extension, another study specified the HPV genotype and showed that the cumulative 2-year risks of ≥CIN 2 in women with <CIN2 were quite different according to HPV types: 27.4% for HPV-16, but 7.5% for other carcinogenic HPV types [12].
Researchers have also attempted to identify epidemiologic characteristics, biomarkers, and immunologic factors that were involved in the progression. In a population-based study in Costa Rica, as a landmark study, Hildesheim et al. [17] compared women with and without ≥HSIL in an HPV-positive group and found several factors that were associated with risk of ≥HSIL, such as increasing number of live births, smoking amount, and no use of barrier contraceptives. In laboratory research, p16 and Ki-67 have received attention as useful biomarkers [18], and in addition, a number of immunologic materials have been examined to identify their role on cervical lesions by affecting the persistence or regression of HPV [19-21].
However, since the results of not only the demographic characteristics but also the specific HPV type and possibly the laboratory measurements are different in accordance with the population, we seek to determine the precise rate of progression in cervical pre-invasive lesions and the associated factors in the Korean population through the cohort study. Finally, this research may ultimately be helpful in the management of pre-invasive cervical diseases and in the primary prevention of cancers and other diseases caused by HPV infection.
Figures and Tables
Fig. 1
Schematic flow chart for the screening and follow-up. Women will be examined by repeat cytology and HPV testing every 6 months. Compared with the previous results of the HPV testing, the next follow-up would yield four possible kinds of change in HPV infection type and the result of cytology will be categorized into two groups (<HSIL and ≥HSIL). ASCUS, atypical squamous cells of undetermined significance, LSIL, low-grade squamous intraepithelial lesion; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion.
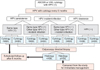
Table 1
Salient description of the procedure interval during the five years of follow-up

HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion.
*During the follow-up, colposcopy and biopsy are not mandatory but can be performed selectively based on the clinician's decision in the case of the mild lesions of <HSIL on cytology. However, if the cytology reveals HSIL or worse, colposcopy and biopsy are necessary.
ACKNOWLEDGMENTS
This study was supported by a grant of Korea Centers for Disease Control and Prevention (no. 2012-E51005-00).
Notes
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Globocan 2008: cancer incidence and mortality worldwide. 2010. Lyon: International Agency for Research on Cancer.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012. 62:10–29.
3. Korean Cancer Registration and Statistics. Cancer incidence and death, 2009 [Internet]. c2012. cited 2012 Dec 10. Goyang: Korea Cancer Registration and Statistics, National Cancer Center;Available from: http://ncc.re.kr/manage/manage03_033_view.jsp?bbsnum=209&hSelSearch=&hTxtKeyword=¤t_page=1&cd=null.
4. Kim JY, Nam BH, Lee JA. Is human papillomavirus genotype an influencing factor on radiotherapy outcome? Ambiguity caused by an association of HPV 18 genotype and adenocarcinoma histology. J Gynecol Oncol. 2011. 22:32–38.
5. Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008. 110:S4–S7.
6. Alvarez RD, Wright TC. Optical Detection Group. Effective cervical neoplasia detection with a novel optical detection system: a randomized trial. Gynecol Oncol. 2007. 104:281–289.
7. Pothisuwan M, Pataradool K, Tangjitgamol S, Srijaipracharoen S, Manusirivithaya S, Thawaramorn T. Visual inspection with acetic acid for detection of high grade lesion in atypical squamous cells and low grade squamous intraepithelial lesions from cervical Pap smear. J Gynecol Oncol. 2011. 22:145–151.
8. Dunn TS, Burke M, Shwayder J. A "see and treat" management for high-grade squamous intraepithelial lesion pap smears. J Low Genit Tract Dis. 2003. 7:104–106.
9. Cox JT, Schiffman M, Solomon D. ASCUS-LSIL Triage Study (ALTS) Group. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003. 188:1406–1412.
10. Reid R, Scalzi P. Genital warts and cervical cancer VII An improved colposcopic index for differentiating benign papillomaviral infections from high-grade cervical intraepithelial neoplasia. Am J Obstet Gynecol. 1985. 153:611–618.
11. Kelsey JL, Whittemore AS, Evans AS, Thompson WD. Methods in observational epidemiology. 1996. 2nd ed. New York: Oxford University Press.
12. Wheeler CM, Hunt WC, Schiffman M, Castle PE. Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study Group. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. J Infect Dis. 2006. 194:1291–1299.
13. Pretorius RG, Peterson P, Azizi F, Burchette RJ. Subsequent risk and presentation of cervical intraepithelial neoplasia (CIN) 3 or cancer after a colposcopic diagnosis of CIN 1 or less. Am J Obstet Gynecol. 2006. 195:1260–1265.
14. Chen EY, Tran A, Raho CJ, Birch CM, Crum CP, Hirsch MS. Histological 'progression' from low (LSIL) to high (HSIL) squamous intraepithelial lesion is an uncommon event and an indication for quality assurance review. Mod Pathol. 2010. 23:1045–1051.
15. Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993. 12:186–192.
16. Walker JL, Wang SS, Schiffman M, Solomon D. ASCUS LSIL Triage Study Group. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS). Am J Obstet Gynecol. 2006. 195:341–348.
17. Hildesheim A, Herrero R, Castle PE, Wacholder S, Bratti MC, Sherman ME, et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br J Cancer. 2001. 84:1219–1226.
18. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001. 25:884–891.
19. Stankiewicz E, Prowse DM, Ng M, Cuzick J, Mesher D, Hiscock F, et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS One. 2011. 6:e17517.
20. Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J, et al. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol. 2011. 224:484–495.
21. Abba MC, Villaverde LM, Gomez MA, Dulout FN, Laguens MR, Golijow CD. The p53 codon 72 genotypes in HPV infection and cervical disease. Eur J Obstet Gynecol Reprod Biol. 2003. 109:63–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download