Abstract
Objective
The aim of the present study was to assess prognostic factors for patients with locally advanced cervical cancer treated with radiotherapy as the primary treatment and to assess the posttreatment cut-off levels of squamous cell carcinoma antigen (SCC-Ag) to predict three-year overall survival (OS) rates.
Methods
One hundred and twenty-eight patients with cervical squamous cell carcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage IIB-IVA) treated using radiotherapy or concurrent chemoradiotherapy were identified. Of these patients, 116 who had SCC-Ag levels >1.5 ng/mL prior to treatment were analyzed retrospectively.
Results
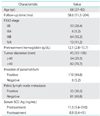
Median age was 68 years (range, 27 to 79 years). The complete response rate was 70.7% and the three-year OS rate was 61.1%. The median levels of pretreatment and posttreatment SCC-Ag were 11.5 ng/mL (range, 1.6 to 310.0 ng/mL) and 0.9 ng/mL (range, 0.4 to 41.0 ng/mL), respectively. Multivariate analysis showed that pretreatment anemia (p=0.041), pelvic lymph node metastasis (p=0.016) and posttreatment SCC-Ag levels (p=0.001) were independent prognostic factors for three-year OS. The SCC-Ag level cut-off point for three-year OS rates, calculated using a receiver operating characteristic curve, was 1.15 ng/mL (sensitivity, 80.0%; specificity, 74.0%).
The squamous cell carcinoma antigen (SCC-Ag) is a glycoprotein with a molecular weight of approximately 45 kDa [1] and a maximum biological half-life of 24 hours [2]. Elevated SCC-Ag levels are found in association with squamous cell carcinomas including cancers of the head and neck, lung, esophagus, anal canal, penis, urethra, and skin. It may also be elevated in other squamous cell gynecological malignancies, such as vulvar and vaginal cancers, benign and immature teratomas with squamous cell elements, and less consistently elevated in female reproductive tract adenocarcinomas. Baseline serum SCC-Ag levels in cervical carcinomas have been successfully correlated with a number of risk factors and tumor characteristics. The release of SCC-Ag into the serum depends on the infiltrative growth and mass of cervical cancer [3]. Baseline levels correlated well with the disease stage, with 30%-40% of stage I patients, 60%-70% of stage II patients, and 80%-90% of stage III-IV patients having elevated values [4-6]. Tumor histology and morphology are also related to SCC-Ag levels because squamous cell and adenosquamous tumors are more likely to elevate baseline levels than pure adenocarcinomas [7].
Serum SCC-Ag has been investigated for other purposes such as monitoring treatment responses or detecting recurrence in patients with cervical cancer. Serum SCC-Ag levels correlate with the extent of disease [8-10], radiotherapy (RT) [11] and chemotherapy [12,13] responses, and can be used to predict survival and tumor recurrence rates during follow-up examinations [14-18]. However, few studies have focused on posttreatment SCC-Ag cut-off levels to predict prognoses.
In the present study, we investigated prognostic factors for patients with locally advanced cervical cancer treated with RT or concurrent chemoradiotherapy (CCRT) as a primary treatment and to assess posttreatment SCC-Ag cut-off levels to predict three-year survival rates.
Between January 1997 and December 2007, 128 patients with cervical squamous carcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage IIB-IVA) undergoing RT or CCRT at the Nara Medical University Hospital were identified. Of these, 116 patients who had pretreatment SCC-Ag levels of >1.5 ng/mL (SCC-Ag positive rate, 90.6%), were analyzed retrospectively. Twelve patients with SCC-Ag levels ≤1.5 ng/mL were excluded from the study. Serum SCC-Ag levels were measured using an immunoradiometric assay with a commercially available kit (SCC-RIA Bead, SRL Inc., Tokyo, Japan). Pretreatment evaluations consisted of complete medical histories, physical examinations, full blood counts, biochemical profiles, serum SCC-Ag levels, cystoscopies, rectosigmoidoscopies, magnetic resonance imagings (MRIs), and computed tomographies (CTs). Lymph nodes >1.0 cm in diameter were interpreted as being involved. Patients with para-aortic lymph node enlargement were excluded. All patients gave their written informed consent for treatment. The patients were treated with anterior-posterior and posteroanterior parallel-opposed ports of external beam radiotherapy (EBRT). The EBRT dose was 50 Gy delivered in 25 fractions. The center shields (4 cm width at the midline) were set up after delivering 40 Gy. The radiation field included the primary tumor, uterus, pelvic lymph node and the paracervical, parametrial, and uterosacral regions.
High-dose intracavitary RT was delivered once per week with a fraction dose of 6.0 Gy at point A three or four times. Since 2000, patients received 40 mg/m2 of cisplatin every week concomitantly with RT. The median cycle of chemotherapy was 5 (range, 3 to 6). A month after the completion of RT or CCRT, all patients were given pathological examination and pelvic MRIs, and were assayed for serum SCC-Ag levels. A measurement of 1.5 ng/mL was considered the normal upper limit. Clinical tumor response was assessed according to the Response Evaluation Criteria in Solid Tumours (RECIST) ver. 1.1. Pathologic response was defined according to the TNM classification. In particular, pathological complete response (CR) was defined as the absence of any residual tumor at a month after the completion of treatment, and pathological partial response (PR) was defined as the persistence of residual tumor.
A gynecologist followed the patients for one month after treatment, then every three months for the first two years, and every 3-6 months thereafter. The follow-up intervals varied for patients suspected of having recurrent diseases, on the basis of individual situations. Patients with suspicious symptoms and signs at physical examination or elevated serum SCC-Ag levels during follow-up periods, underwent additional tests (cytological or pathological examination, abdominopelvic CT scans, pelvic MRI, etc.) to confirm the presence of recurrent diseases.
Kaplan-Meier life table analyses and log-rank tests were used to assess the survival rates and differences based on prognostic factors. Overall survival (OS) was defined as the time from the end of treatment to the last follow-up examination or death. Progression-free survival (PFS) was defined as the time from the end of treatment to any disease progression (local recurrence or distant metastasis). Multivariate analysis of the prognostic factors for OS and PFS was performed using the Cox proportional hazards regression model. Receiver operating characteristic (ROC) curves were used to determine the best cut-off points for serum SCC-Ag levels to predict three-year survival rates. The analysis of the distribution of cases presenting with the serum SCC-Ag levels at a month after treatment according to clinicopathological response was performed by the chi-square test. All statistical testing was conducted at the 0.05 confidence level with SPSS ver.11.0 (SPSS Inc., Chicago, IL, USA).
Disease stage was determined on the basis of the FIGO classifications. The study included 116 patients with FIGO stage IIB-IVA cervical cancer. The median age was 68 years (range, 27 to 79 years). Thirty-three patients had stage IIB, 6 patients had stage IIIA, 64 patients had stage IIIB, and 13 patients had stage IVA. The median follow-up time was 58.6 months (range, 11.3 to 204 months). The median level of pretreatment hemoglobin was 12.1 g/dL (range, 2.8 to 15.7 g/dL). The median level of pretreatment SCC-Ag was 11.5 ng/mL (range, 1.6 to 310 ng/mL), and posttreatment SCC-Ag decreased significantly to 0.9 ng/mL (range, 0.4 to 41.0 ng/mL). Other clinicopathological characteristics are summarized in Table 1. Treatment modalities for cervical cancer patients by FIGO stage are shown in Table 2. For primary therapy, 57 patients received RT, and 59 patients received CCRT. Eighty-five patients (73.3%) had normalized SCC-Ag levels at one month after completing RT or CCRT treatment.
The CR rate was 70.7%. After completion of RT or CCRT, 55 patients (47.4%) experienced tumor recurrence during follow-up examinations. Overall, the three-year PFS and OS rates were 55.2%, and 71.1%, respectively. The Kaplan-Meier method was used to assess the importance of selected factors (i.e., age, FIGO stage, pretreatment hemoglobin levels, cervical tumor diameter, parametrial invasion, pelvic lymph node metastasis and CCRT) influencing the PFS and OS of patients with cervical cancer. In the univariate analysis, FIGO stage (p=0.041), pretreatment hemoglobin levels <10.5 g/dL (p=0.001), tumor size ≥40 mm (p=0.001), pelvic lymph node metastasis (p=0.001) and CCRT (p=0.016) were shown to be associated with OS (Table 3). When Cox multivariate analysis was applied, pelvic lymph node metastasis (p=0.016) and pretreatment hemoglobin levels <10.5 g/dL (p=0.041) were independent prognostic factors for OS (Table 3). Similar results were obtained when examining PFS. Tumor diameter, pretreatment hemoglobin levels <10.5 g/dL and pelvic lymph node metastasis were found to have significant effects on PFS in univariate analysis (p=0.001) (Table 4). Of these, pelvic lymph node metastasis (p=0.017) and pretreatment hemoglobin levels <10.5 g/dL (p=0.001) were significant prognostic factors in multivariate analysis (Table 4).
The cut-off value of SCC-Ag levels at a month after the completion of treatment for the three-year OS rate was 1.15 ng/mL (sensitivity, 80.0%; specificity, 74.0%) (Fig. 1). The three-year OS rates for patients with serum SCC-Ag levels <1.15 ng/mL and ≥1.15 ng/mL were 90.7% and 36.6%, respectively (p<0.001) (Fig. 2). The three-year PFS rates for patients with serum SCC-Ag levels <1.15 ng/mL and ≥1.15 ng/mL were 74.7% and 19.5%, respectively (p<0.001) (Fig. 3).
The cut-off values of SCC-Ag levels for the three-year OS rate in RT and CCRT group were 1.20 ng/mL (area under the curve [AUC], 0.844; 95% confidence interval [CI] 0.740-0.947; sensitivity, 75.0%; specificity, 76.5%) and 1.15 ng/mL (AUC, 0.712; 95% CI, 0.562-0.863; sensitivity, 53.3%; specificity, 83.7%), respectively. The three-year OS rates in RT group with serum SCC-Ag levels <1.20 ng/mL and ≥1.20 ng/mL were 84.3% and 30.8%, respectively (p<0.001). The three-year OS rates in CCRT group with serum SCC-Ag levels <1.15 ng/mL and ≥1.15 ng/mL were 95.1% and 46.7%, respectively (p<0.001).
Eighty-one patients (69.8%) had a clinical CR to RT or CCRT at a month after the completion of treatment, while 35 patients (30.2%) achieved a clinical PR. The three-year OS rates for the patients who achieved clinical CR and clinical PR were 88.8% and 27.1%, respectively (p<0.001). As far as pathological response is concerned, it was available in all cases. pathological CR was documented in 75 cases (64.7%), while pathological PR shown in 41 cases (35.3%). The three-year OS rates for the patients who achieved pathological CR and pathological PR were 81.3% and 6.1%, respectively (p<0.001).
Furthermore, the relationship between serum SCC-Ag levels and the response to treatment was analyzed. The serum SCC-Ag levels <1.15 ng/mL at a month after treatment correlated with the rate of clinical and pathological response (Table 5).
Fifty-five patients of 116 patients (47.4%) were diagnosed with recurrence. Nineteen patients had distant failure, of which 16 had distant metastases, and three had both distant and locoregional recurrence, and the remaining 36 patients had locoregional recurrences. Median survival time after recurrence was 17.4 months (range, 2 to 85.9 months). Only 10 patients with recurrence survived. Death occurred in 29 (49.2%) of 59 patients who received only RT as part of their primary treatment, which was more frequent compared to patients who received CCRT (28.1%) (p=0.016). However, this significance was not present in the multivariate analysis (p=0.085).
Cervical cancer is one of the most common cancers, accounting for 6% of all malignancies in women. Cervical cancer prognosis is affected by disease progression at the time of diagnosis. Until 1998, standard primary therapy for the patients with FIGO stage IIB-IVA cervical cancer consisted of RT without chemotherapy. From 1999 to the present, standard primary treatment consists of CCRT [19-23]. CCRT with cisplatin reduces the relative risk of death from cervical cancer by 30%-50% by decreasing recurrence. CCRT showed significant benefits for local recurrences and may benefit for distant recurrences. However, over 50% of patients with recurrences were found to have distant metastases following CCRT. It is therefore important to identify prognostic factors in patients with locally advanced cervical cancer treated with RT or CCRT.
Several prognostic factors influencing cervical cancer patient survival rates have been established. Some of these factors are related to the patients' characteristics, and the others are related to tumor or treatment characteristics. Pelvic and para-aortic nodal status are the most significant factors in patients with locally advanced cervical cancer [24,25]. We have found that enlarged (>10 mm) lymph nodes found on CT/MRI before treatment are indicative of a shorter survival period and increased disease progression in cervical cancer patients. Univariate and multivariate analyses showed that pelvic node metastasis assessed using CT/MRI was one of the most important prognostic factors in RT or CCRT of cervical cancer.
The clinical importance of pretreatment anemia on prognosis following curative RT was first identified in cervical cancer patients treated during the early 1940s [26]. The negative impact of pretreatment anemia on postradiation locoregional control and survival has been well documented in patients with squamous cell carcinoma of the uterine cervix [27-29]. In our study population, pretreatment anemia (hemoglobin levels <10.5 g/dL) was found to be an independent prognostic predictor by univariate and multivariate analyses.
In studies of patients treated primarily by radical surgery, increased pretreatment SCC-Ag levels were found to correlate unfavorably with clinicopathological characteristics including advanced stage and larger tumor size, lymph node metastasis or parametrial involvement [30,31], deep stromal infiltration, and blood-vessel invasion [32]. There were many studies showing that SCC-Ag levels are a suitable serum marker for monitoring tumor treatment response and relapse detection. Ngan et al. [11] found that SCC-Ag levels at the end of external RT (before intracavity brachytherapy) had no clinical significance on tumor control. However, persistently high SCC-Ag levels following RT completion were significantly related to the residual tumor [11,32]. Some authors have shown that normalization of elevated SCC-Ag levels was associated with a complete response in cervical cancer [14,33]. In our study, the serum SCC-Ag levels at a month after treatment correlated with the rate of clinical and pathological response. These findings suggest that the serum SCC-Ag levels at a month after treatment could be an indicator of the tumor response in patients with uterine cervical cancer.
Yoon et al. [34] reported that pretreatment SCC-Ag levels were associated with the FIGO stage, tumor volume, and lymph node status, and one month after completing CCRT, the SCC-Ag levels was normalized in almost all patients with an incidence of 93.2%. Hirakawa [27] described that positive serum SCC-Ag levels, immediately after CCRT, is a significant predictor of distant recurrence in patients with locally advanced cervical squamous cell carcinoma treated with CCRT and helpful in identifying those patients who are at the risk of distant metastases.
Hong et al. [14] reported that the five-year disease-specific survival rates for patients with normal and abnormally high SCC-Ag values (>2.0 ng/mL) after RT were 88% and 17%, respectively, and the five-year disease-specific survival rates were 89%, 85%, 29%, and 0% for patients without residual induration/SCC-Ag levels of <2.0 ng/mL, with residual induration/SCC-Ag levels of <2.0 ng/mL, without residual induration/SCC-Ag levels of >2.0 ng/mL and with residual induration/SCC-Ag levels of >2.0 ng/mL, respectively. In other words, patients with posttreatment residual induration, but normal SCC-Ag levels, still had relatively good prognoses, suggesting that elevated posttreatment SCC-Ag levels are a stronger predictor of poor outcome than residual induration [14]. According to our ROC analysis, the area under the ROC curve indicated that posttreatment SCC-Ag levels averaged 1.15 ng/mL (sensitivity, 80.0%; specificity, 74.0%) for patients who had been treated with RT or CCRT. Therefore, our results indicated that posttreatment SCC-Ag levels had good clinical performances as prognosis predictors.
In order to reduce the relapse after RT or CCRT, the strategy to perform some adjuvant therapy for high-risk cases may be considered. So, there is a possibility that serum SCC-Ag level of posttreatment is useful for screening high-risk group suitable to adjuvant therapy.
In conclusion, with the limits inherent in the retrospective nature of this series, we reported that pretreatment anemia and positive results for pelvic lymph node metastasis are independent prognostic factors in locally advanced cervical cancer. Furthermore, posttreatment SCC-Ag of levels <1.15 ng/mL predicted better three-year survival rates. We suggest that clinicians consider performing routine serum SCC-Ag measurements after treatment. These data provide further evidence of the clinical usefulness of SCC-Ag assessment in cervical cancer and call for large and prospective studies.
Figures and Tables
Fig. 1
Receiver operating curve for posttreatment serum squamous cell carcinoma antigen in predicting 3-year survival rate in patients treated with radiotherapy or concurrent chemoradiotherapy. AUC, area under the curve; CI, confidence interval.

Fig. 2
Overall survival depending on the posttreatment squamous cell carcinoma antigen (SCC-Ag) level.

Fig. 3
Progression-free survival depending on the posttreatment squamous cell carcinoma antigen (SCC-Ag) level.

References
1. Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977; 40:1621–1628.
2. Schmidt-Rhode P, Schulz KD, Sturm G, Hafner H, Prinz H, Kunzig HJ. Squamous cell carcinoma antigen for monitoring cervical cancer. Int J Biol Markers. 1988; 3:87–94.
3. Crombach G, Scharl A, Vierbuchen M, Wurz H, Bolte A. Detection of squamous cell carcinoma antigen in normal squamous epithelia and in squamous cell carcinomas of the uterine cervix. Cancer. 1989; 63:1337–1342.
4. Neunteufel W, Tatra G, Bieglmayer C. Serum squamous cell carcinoma antigen levels in women with neoplasms of the lower genital tract and in healthy controls. Arch Gynecol Obstet. 1989; 246:243–250.
5. Brioschi PA, Bischof P, Delafosse C, Krauer F. Squamous-cell carcinoma antigen (SCC-A) values related to clinical outcome of pre-invasive and invasive cervical carcinoma. Int J Cancer. 1991; 47:376–379.
6. Senekjian EK, Young JM, Weiser PA, Spencer CE, Magic SE, Herbst AL. An evaluation of squamous cell carcinoma antigen in patients with cervical squamous cell carcinoma. Am J Obstet Gynecol. 1987; 157:433–439.
7. Kornafel J, Wawrzkiewicz M. Evaluation of diagnostic usefulness of CEA, hCG and SCC antigens in cervical cancer patients. Eur J Gynaecol Oncol. 1989; 10:319–322.
8. Bolger BS, Dabbas M, Lopes A, Monaghan JM. Prognostic value of preoperative squamous cell carcinoma antigen level in patients surgically treated for cervical carcinoma. Gynecol Oncol. 1997; 65:309–313.
9. Bolli JA, Doering DL, Bosscher JR, Day TG Jr, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994; 55:169–173.
10. Strauss HG, Laban C, Lautenschlager C, Buchmann J, Schneider I, Koelbl H. SCC antigen in the serum as an independent prognostic factor in operable squamous cell carcinoma of the cervix. Eur J Cancer. 2002; 38:1987–1991.
11. Ngan HY, Chan SY, Wong LC, Choy DT, Ma HK. Serum squamous cell carcinoma antigen in the monitoring of radiotherapy treatment response in carcinoma of the cervix. Gynecol Oncol. 1990; 37:260–263.
12. Meier W, Eiermann W, Stieber P, Fateh-Moghadam A, Schneider A, Hepp H. Squamous cell carcinoma antigen and carcinoembryonic antigen levels as prognostic factors for the response of cervical carcinoma to chemotherapy. Gynecol Oncol. 1990; 38:6–11.
13. Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol. 1994; 12:2309–2316.
14. Hong JH, Tsai CS, Chang JT, Wang CC, Lai CH, Lee SP, et al. The prognostic significance of pre- and posttreatment SCC levels in patients with squamous cell carcinoma of the cervix treated by radiotherapy. Int J Radiat Oncol Biol Phys. 1998; 41:823–830.
15. Kato H, Miyauchi F, Morioka H, Fujino T, Torigoe T. Tumor antigen of human cervical squamous cell carcinoma: correlation of circulating levels with disease progress. Cancer. 1979; 43:585–590.
16. Micke O, Bruns F, Schafer U, Prott FJ, Willich N. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-) radiotherapy. Anticancer Res. 2005; 25:1663–1666.
17. Ngan HY, Cheng GT, Yeung WS, Wong LC, Ma HK. The prognostic value of TPA and SCC in squamous cell carcinoma of the cervix. Gynecol Oncol. 1994; 52:63–68.
18. Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002; 52:23–32.
19. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999; 340:1137–1143.
20. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–1613.
21. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340:1144–1153.
22. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999; 17:1339–1348.
23. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999; 340:1154–1161.
24. Yamashita H, Nakagawa K, Tago M, Shiraishi K, Nakamura N, Ohtomo K. Treatment results and prognostic analysis of radical radiotherapy for locally advanced cancer of the uterine cervix. Br J Radiol. 2005; 78:821–826.
25. Grigiene R, Valuckas KP, Aleknavicius E, Kurtinaitis J, Letautiene SR. The value of prognostic factors for uterine cervical cancer patients treated with irradiation alone. BMC Cancer. 2007; 7:234.
26. Evans JC, Bergsjo P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology. 1965; 84:709–717.
27. Hirakawa M, Nagai Y, Inamine M, Kamiyama K, Ogawa K, Toita T, et al. Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Gynecol Oncol. 2008; 108:126–129.
28. Mundt AJ, Connell PP, Campbell T, Hwang JH, Rotmensch J, Waggoner S. Race and clinical outcome in patients with carcinoma of the uterine cervix treated with radiation therapy. Gynecol Oncol. 1998; 71:151–158.
29. Takeshi K, Katsuyuki K, Yoshiaki T, Teppei S, Tadayoshi M, Akira M, et al. Definitive radiotherapy combined with high-dose-rate brachytherapy for Stage III carcinoma of the uterine cervix: retrospective analysis of prognostic factors concerning patient characteristics and treatment parameters. Int J Radiat Oncol Biol Phys. 1998; 41:319–327.
30. Duk JM, Groenier KH, de Bruijn HW, Hollema H, ten Hoor KA, van der Zee AG, et al. Pretreatment serum squamous cell carcinoma antigen: a newly identified prognostic factor in early-stage cervical carcinoma. J Clin Oncol. 1996; 14:111–118.
31. Gaarenstroom KN, Bonfrer JM, Kenter GG, Korse CM, Hart AA, Trimbos JB, et al. Clinical value of pretreatment serum Cyfra 21-1, tissue polypeptide antigen, and squamous cell carcinoma antigen levels in patients with cervical cancer. Cancer. 1995; 76:807–813.
32. Massuger LF, Koper NP, Thomas CM, Dom KE, Schijf CP. Improvement of clinical staging in cervical cancer with serum squamous cell carcinoma antigen and CA 125 determinations. Gynecol Oncol. 1997; 64:473–476.
33. Rose PG, Baker S, Fournier L, Nelson BE, Hunter RE. Serum squamous cell carcinoma antigen levels in invasive cervical cancer: prediction of response and recurrence. Am J Obstet Gynecol. 1993; 168:942–946.
34. Yoon SM, Shin KH, Kim JY, Seo SS, Park SY, Kang S, et al. The clinical values of squamous cell carcinoma antigen and carcinoembryonic antigen in patients with cervical cancer treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2007; 17:872–878.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download