Abstract
Objective
The aim of this study was to investigate the prognostic factors and treatment outcome of patients with adenocarcinoma of the uterine cervix who underwent radical hysterectomy with systematic lymphadenectomy.
Methods
A total of 130 patients with stage IB to IIB cervical adenocarcinoma treated with hysterectomy and systematic lymphadenectomy from 1982 to 2005 were retrospectively analyzed. Clinicopathological data including age, stage, tumor size, the number of positive node sites, lymphovascular space invasion, parametrial invasion, deep stromal invasion (>2/3 thickness), corpus invasion, vaginal infiltration, and ovarian metastasis, adjuvant therapy, and survival were collected and Cox regression analysis was used to determine independent prognostic factors.
Results
An estimated five-year survival rate of stage IB1 was 96.6%, 75.0% in stage IB2, 100% in stage IIA, and 52.8% in stage IIB. Prognosis of patients with one positive-node site is similar to that of those with negative-node. Prognosis of patients with multiple positive-node sites was significantly poorer than that of negative and one positive-node site. Multivariate analysis revealed that lymph node metastasis, lymphovascular space invasion, and parametrial invasion were independent prognostic factors for cervical adenocarcinoma. Survival of patients with cervical adenocarcinoma was stratified into three groups by the combination of three independent prognostic factors.
Adenocarcinoma (ADC) of the uterine cervix is a relatively uncommon histological subtype of cervical cancer, while have been increasing recently in many areas [1]. Several reports have shown that ADC is more aggressive and exhibits more distant metastases, resulting in a lower survival rate than squamous cell carcinoma (SCC) [2,3]. Our incomplete understanding of the prognostic factors [4,5] and optimal treatment [6,7] of ADC may account for its poor outcome. Indeed, there has been no uniformly accepted form of management for ADC. As with SCC, patients with International Federation of Obstetrics and Gynecology (FIGO) stage IB1-IIB cervical ADC are treated by radical hysterectomy (RH). The prognosis of patients with ADC after RH remains unclear and conventional adjuvant therapy in high-risk group after primary surgery or salvage therapy of recurrent ADC seems generally ineffective [6]. Several reports found that patients with ADC have poorer prognosis than do those with SCC [4,5,8-10], whereas others found no differences in prognosis [11-15]. Therefore, the prognosis after RH and the optimal management of ADC are still a matter of debate.
Thus, the aim of this study was to clarify the treatment outcomes and prognostic factors after RH in patients with FIGO stage IB1-IIB ADC of uterine cervix, and to postulate the optimal management of patients with early-stage ADC of uterine cervix.
After approval by the Institutional Review Board of Hokkaido University Hospital, we searched the cancer registry and computerized database of our institution for patients with 1) FIGO stage IB1-IIB cervical ADC, 2) who underwent RH with removal of a vaginal cuff of at least 2 cm, total resection of parametrial tissue and systematic lymphadenectomy (LND). This operation is a nerve-sparing modification of the Okabayashi operation [16]. The nerve-sparing procedure was further refined by introducing the preservation of vesical branches of pelvic plexus since 1997. As the preferred treatment in our institution for patients with FIGO stage IB1-IIB cervical cancer is RH, almost all patients with FIGO stage IB1-IIB cervical cancer underwent RH and only a small number of patients who were not eligible for radical surgery because of severe medical co-morbidity received radiotherapy (RT) or concurrent chemoradiotherapy (CCRT). Medical records were retrospectively reviewed, and the following parameters were collected: age, FIGO stage, tumor size, deep stromal invasion (DSI, >2/3 thickness), parametrial invasion (PI), lymphovascular space invasion (LVSI), lymph node metastasis (LNM), corpus invasion, vaginal metastasis, ovarian metastasis, adjuvant therapy, and date of death or last follow-up. Pathologic slides were reviewed by two experienced pathologists at our institution.
Since there is no definitive evidence that adjuvant RT is more effective than adjuvant chemotherapy for cervical ADC undergoing RH, we have used more frequent adjuvant chemotherapy after surgery since the year 2000 [16]. Patients with risk factors for recurrence, including DSI, LVSI, PI, LNM, and/or bulky tumor, received whole pelvic irradiation or systemic platinum-based chemotherapy as postsurgical adjuvant therapy. RT consisted of whole pelvic external irradiation by four-field technique with 50 Gy for 25 fractions beginning four weeks after surgery. Chemotherapy was given at least three courses at four-week intervals beginning approximate 3 weeks after surgery. Chemotherapeutic regimens used were previously described [17]. Briefly, IEP (ifosphamide: 1.5 g/m2, day 1-3; epirubicin: 40 mg/m2, day 1; cisplatin: 14 mg/m2, day 1-5) was used for eleven patients, CAP (cyclophosphamide: 500 mg/m2, day 1; adriamycin: 50 mg/m2, day 1; cisplatin: 50 mg/m2, day 1) for five, MEP (mitomycin C: 10 mg/body, day 1; cisplatin: 70 mg/m2, day 1; etoposide: 100 mg/m2, day 1-3) for two, BOMP (bleomycin: 7 mg/body, day 1-5; vincristine: 0.7 mg/m2, day 5; mitomycin C: 7 mg/m2, day 5; cisplatin: 14 mg/m2, day 1-5) for one, TC (paclitaxel: 175 mg/m2, day 1; carboplatin: AUC5, day 1) for one, TP (paclitaxel: 135 mg/m2, day 1; cisplatin: 50 mg/m2, day 2) for one, ITP (ifosphamide: 1.5 g/m2, day 1-3; paclitaxel: 175 mg/m2, day 1; cisplatin: 14 mg/m2, day 1-5) for one, and intra-arterial infusion of cisplatin (100 mg/body) for one, and cisplatin-based chemotherapy (unknown) for seven.
Categorical variables were analyzed using the chi-square test or Fisher's exact test. We used the Kaplan-Meier method, log-rank test for survival analysis and the Cox hazard method for prognostic analysis. A result was considered significant when the p-value was less than 0.05.
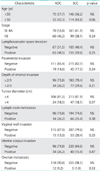
The clinicopathological characteristics of 130 patients with cervical ADC are summarized in Table 1. Among 130 patients with cervical ADC, sixty had clinical stage IB1, six had stage IB2, four had stage IIA, and sixty had stage IIB. Histologic subtypes include eighty-eight cases of endocervical type, two of intestinal type, thirty-two of adenosquamous carcinoma, seven of endometrioid type, and one of clear cell carcinoma. Median age of the patients was 47 years (range, 26 to 69 years). Median follow-up period was 72 months (range, 4 to 120 months). Among 130 patients, seventy-one patients did not receive adjuvant therapy, because 53 patients showed no risk factors for recurrence, and 18 patients refused adjuvant therapy due to personal reasons. Fifty-nine patients with risk factors for recurrence received adjuvant therapy, including 29 cases of RT and 30 cases of systemic platinum-based chemotherapy.
All cases of ovarian metastasis had stage IIB disease. Ovarian metastasis was found in 12 of 130 cases (9%), and 11 of 12 cases died within five years. Ovarian metastasis is significantly related to other pathological risk factors including LN metastasis, LVSI, parametrial invasion, DSI, vaginal invasion, and corpus invasion (p<0.001 for all 6 risk factors), which should explain why the patients with ovarian metastasis showed extremely poor survival (Table 1). We, therefore, excluded ovarian metastasis for multivariate survival analysis in this study (Fig. 1) (Table 2).
An estimated five-year survival rate of stage IB1 was 96.6%, 75.0% in stage IB2, 100% in stage IIA, and 52.8% in stage IIB. Among the clinicopathological factors analyzed in this study, all risk factors except for age are significantly related to poor survival by univariate analysis (Table 3). Multivariate analysis revealed that LNM (hazard ratio [HR], 4.4, 95% confidence interval [CI], 1.7 to 11.4; p=0.002), LVSI (HR, 4.0; 95% CI, 1.1 to 14.1; p=0.03), and PI (HR, 4.6; 95% CI, 1.8 to 11.5; p=0.001) were independent prognostic factors in cervical ADC treated with RH and systematic LND (Table 3).
Survival of the patients with cervical ADC could be stratified into three groups by the combination of three independent prognosticators. An estimated five-year survival rate for the patients without three independent risk factors (group A, n=59) was 98%, that for the patients with LVSI and/or PI without LNM (group B, n=37) was 75%, and that for the patients with LNM irrespective of the presence of LVSI/PI (group C, n=34) was 37%. There is statistically significant difference of disease-specific survival among each group (p=0.004 for group A vs. group B, p<0.001 for group B vs. group C, p<0.001 for group A vs. group C) (Fig. 2).
Since multivariate analysis has shown that LNM was one of the most important prognostic factors in cervical ADC, we analyzed its impact on the survival in cervical ADC according to the number of positive-node sites. Estimated five-year survival rate of the patients without LNM (group A, n=96) was 89%. Estimated five-year survival rate with one positive-node site (group B, n=7), and with more than two positive-node sites (group C, n=27) was 86% and 23%, respectively (Fig. 3). There was statistically significant difference of survival between group A and C (p<0.001), and between group B and C (p=0.009). However, there was no statistically significant difference of survival between group A and B (p=0.29).
In this retrospective analysis, we demonstrated that LNM, LVSI, and PI were independent prognostic factors for ADC of the uterine cervix, and their survival was stratified by the combination of three independent prognostic factors. Postoperative treatment and follow-up modality could be individualized according to the independent risk factors for survival.
Several reports found that patients with ADC have poorer prognosis than do those with SCC [4,5,8-10], whereas others found no differences in prognosis [11-15]. When we compared the frequency of clinicopathologic risk factors and survival between ADC and SCC (260 cases) at our institution during the same study period, we found no significant difference of frequency of clinicopathologic risk factors according to the histologic subtype (Table 4). However, overall survival of ADC was significantly worse than that of SCC (Fig. 4), suggesting that adjuvant therapy might be less effective for ADC than SCC.
Currently, standard adjuvant therapy was not fully established after RH and LND for cervical ADC, but RT is widely employed as a standard adjuvant therapy as well as for SCC. However, there is no definitive evidence that RT is more beneficial than chemotherapy after radical surgery for cervical cancer. Thus, adjuvant chemotherapy combined with RH and systematic LND may also provide a survival benefit. However, there are no randomized controlled studies comparing the clinical efficacy of RT and chemotherapy after surgery so far. We need to analyze the survival difference according to type of adjuvant therapy in other patients' cohort and in randomized trials in the future.
LNM have been shown to be the most important prognosticator for ADC of the uterine cervix. However, it is still unclear whether number of LNM sites affect differential survival of cervical ADC treated with RH and pelvic lymph node dissection. We, therefore, analyzed overall survival according to the number of positive-node sites and demonstrated that survival with one positive-node site was not significantly worse than that with negative-node (Fig. 3), which is similar to our previous report [18]. We speculate that single LNM site might be a local disease, and can be curable by our current treatment strategy consisting of extensive surgery and adjuvant therapy. This result is similar to that in node-positive endometrial cancer as we previously reported [19].
In contrast to node-negative patients, prognosis of patients with multiple positive-node sites was significantly worse than that with no and one positive-node site, indicating that most appropriate treatment strategy for patients with multiple positive-node sites remains to be established. If we can accurately predict LNM preoperatively, we can choose CCRT instead of radical surgery as a primary treatment for patients with multiple positive-node sites. Among risk factors, which can be assessed preoperatively, tumor size is supposed to be a good predictive factor for LNM. However, its prognostic value remains controversial [10,15]. In fact, tumor size was not an independent prognostic factor in our patient cohort. One possible preoperative assessment to predict LNM might be use of the combination of serum tumor markers, because we have previously shown that preoperative serum SCC and CA-125 levels strongly associated with number of positive pelvic nodes, site of positive-node in cervical SCC [20]. Alternatively, it is worth to utilize new imaging technique to efficiently detect LNM. Magnetic resonance/positron emission tomography (PET) has been reported to be more accurate than PET-computed tomography (CT) to predict LNM in cervical cancer [21].
In summary, we found three independent prognostic factors for patients with ADC of the uterine cervix who underwent RH and systematic LND. Prospective study is necessary to establish standard primary treatment (CCRT or RH) or standard adjuvant therapy (RT or CCRT or chemotherapy) after RH and systematic LND for early-stage ADC of the uterine cervix with multiple-node sites to improve their survival.
Figures and Tables
Fig. 1
Survival of adenocarcinoma of the uterine cervix according to ovarian metastasis. All cases of ovarian metastasis had stage IIB disease. Ovarian metastasis was found in 12 of 130 cases (9%), and 11 of 12 cases died within five years.

Fig. 2
Stratification of survival of the patients with cervical adenocarcinoma by the combination of three independent prognosticators. An estimated five-year survival rate for the patients without three independent risk factors was 98%, that for the patients with lymphovascular space invasion (LVSI) and/or parametrial invasion (PI) without lymph node metastasis (LNM) was 75%, and that for the patients with LNM irrespective of the presence of LVSI/PI was 37%.

Fig. 3
Impact of lymph node metastasis (LNM) on the survival of adenocarcinoma of the uterine cervix. Estimated five-year survival rate of the patients without LNM (group A) was 89%, that with one positive-node site (group B), and that with more than two positive-node sites (group C) was 86% and 23%, respectively.

Fig. 4
Comparison of overall survival according to the histologic type. ADC, adenocarcinoma; SCC, squamous cell carcinoma.

References
1. Schorge JO, Knowles LM, Lea JS. Adenocarcinoma of the cervix. Curr Treat Options Oncol. 2004; 5:119–127.
2. Look KY, Brunetto VL, Clarke-Pearson DL, Averette HE, Major FJ, Alvarez RD, et al. An analysis of cell type in patients with surgically staged stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1996; 63:304–311.
3. Eifel PJ, Burke TW, Morris M, Smith TL. Adenocarcinoma as an independent risk factor for disease recurrence in patients with stage IB cervical carcinoma. Gynecol Oncol. 1995; 59:38–44.
4. Brand E, Berek JS, Hacker NF. Controversies in the management of cervical carcinoma. Obstet Gynecol. 1988; 71:261–269.
5. Goodman HM, Buttlar CA, Niloff JM, Welch WR, Marck A, Feuer EJ, et al. Adenocarcinoma of the uterine cervix: prognostic factors and patterns of recurrence. Gynecol Oncol. 1989; 33:241–247.
6. Lai CH, Hsueh S, Hong JH, Chang TC, Tseng CJ, Chou HH, et al. Are adenocarcinomas and adenosquamous carcinomas different from squamous carcinomas in stage IB and II cervical cancer patients undergoing primary radical surgery? Int J Gynecol Cancer. 1999; 9:28–36.
7. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–540.
8. Kim SM, Choi HS, Byun JS. Overall 5-year survival rate and prognostic factors in patients with stage IB and IIA cervical cancer treated by radical hysterectomy and pelvic lymph node dissection. Int J Gynecol Cancer. 2000; 10:305–312.
9. Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000; 79:289–293.
10. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer. 2010; 102:1692–1698.
11. Grisaru D, Covens A, Chapman B, Shaw P, Colgan T, Murphy J, et al. Does histology influence prognosis in patients with early-stage cervical carcinoma? Cancer. 2001; 92:2999–3004.
12. Ayhan A, Al RA, Baykal C, Demirtas E, Yuce K, Ayhan A. A comparison of prognoses of FIGO stage IB adenocarcinoma and squamous cell carcinoma. Int J Gynecol Cancer. 2004; 14:279–285.
13. Lee KB, Lee JM, Park CY, Lee KB, Cho HY, Ha SY. What is the difference between squamous cell carcinoma and adenocarcinoma of the cervix? A matched case-control study. Int J Gynecol Cancer. 2006; 16:1569–1573.
14. Fregnani JH, Soares FA, Novik PR, Lopes A, Latorre MR. Comparison of biological behavior between early-stage adenocarcinoma and squamous cell carcinoma of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2008; 136:215–223.
15. Kasamatsu T, Onda T, Sawada M, Kato T, Ikeda S, Sasajima Y, et al. Radical hysterectomy for FIGO stage I-IIB adenocarcinoma of the uterine cervix. Br J Cancer. 2009; 100:1400–1405.
16. Hosaka M, Watari H, Takeda M, Moriwaki M, Hara Y, Todo Y, et al. Adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy for patients with cervical squamous cell carcinoma. J Obstet Gynaecol Res. 2008; 34:552–556.
17. Watari H, Kanuma T, Ohta Y, Hassan MK, Mitamura T, Hosaka M, et al. Clusterin expression inversely correlates with chemosensitivity an predicts poor survival in patients with locally advanced cervical cancer treated with cisplatin-based neoadjuvant chemotherapy and radical hysterectomy. Pathol Oncol Res. 2010; 16:345–352.
18. Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand. 2002; 81:1144–1151.
19. Watari H, Todo Y, Takeda M, Ebina Y, Yamamoto R, Sakuragi N. Lymph-vascular space invasion and number of positive paraaortic node groups predict survival in node-positive patients with endometrial cancer. Gynecol Oncol. 2005; 96:651–657.
20. Takeda M, Sakuragi N, Okamoto K, Todo Y, Minobe S, Nomura E, et al. Preoperative serum SCC, CA125, and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta Obstet Gynecol Scand. 2002; 81:451–457.
21. Kim SK, Choi HJ, Park SY, Lee HY, Seo SS, Yoo CW, et al. Additional value of MRI/PET fusion vompared with PET-CT in the detection of lymph node metastasis in cervical cancer patients. Eur J Cancer. 2009; 45:2103–2109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download