Abstract
A 35-year-old woman underwent laparoscopic radical hysterectomy, pelvic lymphadenectomy and ovarian transposition for stage IB2 cervical adenocarcinoma. She received adjuvant concurrent chemoradiation for poor pathologic risk factors but had tumor recurrence 20 months after the surgery. Transposed ovaries were uninvolved in the recurrence and progression. Salvage chemotherapy and radiotherapy were given. Despite systemic chemotherapy and repeat pelvic radiotherapy, the patient was able to maintain ovarian function. Ovarian transposition in cervical cancer is an easily performed procedure that does not alter the prognosis of the disease in some cases. Present recommendations for its use should be reevaluated so that more premenopausal cancer patients may benefit from this underutilized procedure.
Ovarian transposition has proven to be an invaluable procedure in the maintenance of ovarian function in the premenopausal patient undergoing radiotherapy. The criteria for ovarian transposition in cervical cancer, though not standardized throughout the world, generally include squamous cell, early-stage tumors with a need for adjuvant radiotherapy. The rationale for these conservative criteria is unrecognized ovarian metastasis in the retained ovaries.
Herein we describe a case of a 35-year-old patient who underwent radical hysterectomy with ovarian transposition for cervical adenocarcinoma. Although tumor recurrence and progression occurred despite surgico-radiotherapy, the patient was able to maintain some ovarian function with the ovaries uninvolved in the recurrence.
A 35-year-old gravida 4 para 0 Taiwanese woman diagnosed with stage IB2 cervical adenocarcinoma underwent total laparoscopic radical hysterectomy, bilateral salpingectomy, pelvic lymph node dissection and ovarian transposition in June 2009. It is the practice of the Gynecologic Oncology Service of Chang Gung Memorial Hospital to perform radical hysterectomy for patients with bulky cervical adenocarcinoma. She had no concomitant illnesses or family history of breast or ovarian cancer. Previous menstrual cycles were normal except for intermenstrual bleeding that occurred four months prior to the diagnosis. Baseline pelvic examination revealed an exophytic cervical mass measuring over 4 cm, with smooth parametria. Preoperative tumor markers squamous cell carcinoma (0.8 ng/mL), carcinoembryonic antigen (CEA, 1.9 ng/mL) and CA-125 (24.3 U/mL) were within normal limits.
She underwent ovarian transposition during radical hysterectomy. Five trocars were inserted for four handed surgery with the primary trocar placed at Lee-Huang point (midway between the umbilicus and the xiphoid process). Grossly normal ovaries were fixed retroperitoneally at the antero-lateral abdominal wall, 3 cm above the umbilicus, below the upper trocars on each side. Metal clips were placed on the pedicles of both ovaries for radiologic detection.
The uterus measured 9.5×6.5×3.3 cm with an exophytic cervical mass measuring 7.5×4.3×1.8 cm. Vaginal length was 1.7 cm with right and left parametria measuring 3×1.5×1.5 cm and 3.5×2.8×2 cm, respectively. On microscopic examination, there was full cervical stromal invasion and involvement of the endometrium. There was focal lymphovascular space invasion. All surgical margins were negative. One of out seven left pelvic lymph nodes was positive for metastasis. Tumor was focally positive for CEA but negative for estrogen receptor, progesterone receptor, and vimentin. Final histopathology was moderately differentiated cervical adenocarcinoma. She received five cycles of weekly cisplatin at 40 mg/m2 concurrent with external beam radiotherapy to the whole pelvis and true pelvis at a dose of 4,050 cGy and 5,040 cGy, respectively, and underwent three sessions of brachytherapy, 200 cGy/fraction (Fig. 1).
Imaging, papanicolau smear and tumor markers post-treatment were normal. One year and 3 months after ovarian transposition, estradiol was <7 pg/mL, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were elevated at 95.9 mIU/mL and 139.1 mIU/mL, respectively. She had no complaints of hot flashes. Five months later, the patient experienced low back pain radiating to the right thigh. Pelvic computed tomography revealed tumor recurrence at the left vaginal stump, bilateral pelvic sidewalls and right common iliac bifurcation with severe right hydronephrosis. Computed tomography (CT)-guided biopsy of the pelvic mass showed metastastic adenocarcinoma. She received cisplatin 50 mg/m2 (day 3) and topotecan 0.75 mg/m2 (day 1-3) for 6 cycles as palliative chemotherapy. She underwent external beam radiotherapy to the pelvis for pain control with a dose of 2,500 cGy/10 fractions at the same period. Two years and three months after the initial surgery, estradiol was 30.41 pg/mL, LH 0.2 mIU/mL, and FSH 5.6 mIU/mL. Repeat CT scan at this time showed transposed ovaries with metallic clips remained fixed to the anterior abdominal wall and uninvolved in tumor recurrence. Ultrasonography revealed both ovaries to be normal in size (Fig. 2). After two months, estradiol decreased to 13.5 pg/mL, and LH and FSH remained low, at <0.1 mIU/mL and 1.6 mIU/mL, respectively.
Bilateral percutaneous nephrostomy was performed for azotemia secondary to tumor progression. At present, the patient is receiving supportive palliative care. On her last follow-up, 2 years and 9 months after ovarian transposition, estradiol was 23.5 pg/mL, LH 1.97 U/L, and FSH 1.4 U/L.
The two main considerations in performing ovarian transposition in cervical cancer are efficacy and safety. Success rates as high as 90% have been reported, although increased doses of radiation, especially if combined with chemotherapy, increase the rate of ovarian failure [1].
The issue of oncologic safety hinges on cervical cancer being a hormonally-independent tumor with a relatively low incidence of ovarian metastasis. Several risk factors for ovarian involvement have been identified. Non-squamous histology portends a higher risk for ovarian metastasis than squamous histology, with an incidence of 2.4% versus 0.5%, respectively, in early-stage cervical carcinoma [2]. Other known risk factors are tumor size, cervical stromal invasion, and lymphovascular space invasion [2-4].
Yamamoto's criteria for transposition include an age of 44 years old or younger with normal preoperative ovarian function, no macroscopic abnormalities on inspection of the ovaries, FIGO (International Federation of Gynecology and Obstetrics) stage IB2, a candidate for postoperative radiotherapy and no history of breast or ovarian cancer [3]. Most authors advocate an age of less than 40 years old because of the higher risk of ovarian failure beyond this age [1]. The cut-off of tumor size ranges from less than 3-4 cm [1,2]. Some believe non-squamous histology is a deterrent to ovarian transposition [2]. Others advocate that lymphovascular space invasion, cervical stromal invasion and uterine corpus involvement as contraindications [2,5]. The rationale remains the same, which is to prevent retention of ovaries with metastasis undiscovered during the time of surgery. In effect, the procedure is limited to a population of young patients with early-staged, small, operable tumors with a need for adjuvant radiotherapy. Patients with locally-advanced disease or with poor prognostic factors with a high risk of recurrence are not candidates for the procedure.
A retrospective study by Han et al. [6] in a national university hospital showed the underutilization of ovarian transposition. The procedure was performed in only 28.7% (31/108) of patients aged 12-40 years old who would undergo primary or adjuvant pelvic radiation. The most common indication was cervical cancer, which ranged from stage IB1 to IIB. All but one case was done via laparotomy. They recommend that gynecologic oncologists should discuss with patients the benefits of ovarian transposition to increase awareness and its use [6].
In our patient we performed ovarian transposition during laparoscopic radical hysterectomy despite a bulky 7.5-cm cervical adenocarcinoma. Final histopathology showed lymph node metastasis, full cervical stromal invasion, uterine corpus involvement and lymphovascular space invasion, all of which are risk factors for ovarian metastasis, as well as tumor recurrence [7]. In less than two years, the patient's disease returned despite primary surgical treatment and adjuvant chemo-radiotherapy. Recurrence occurred in the pelvis specifically in the vaginal stump, the most common site post radical hysterectomy [8]. By CT, the tumor extended to the pelvic sidewalls and great iliac vessels, but with no ovarian involvement. In this regard, we believe that performing ovarian transposition did not affect the course of the disease.
Approximately a year after primary surgery and chemoradiotherapy, our patient's serum FSH and LH were markedly elevated and estradiol level was low, which was consistent with menopause. She then received systemic chemotherapy and pelvic radiotherapy for tumor recurrence. Repeat FSH and LH dropped to 5.6 and 0.2 mIU/mL, respectively, and remained consistently low two months later. The initial rise and subsequent fall of FSH and LH may be explained by the phenomenon of transient ovarian failure. Described in some reports but not fully elucidated, ovarian function may resume after exposure to radiation, chemotherapy or devascularization after surgery. It is proposed that inactive follicles at the time of the injury may resume their cycles and responsiveness to gonadotropins months after removal of the insult [9,10]. Although more concrete measures of retained ovarian function would be resumption of menses and pregnancy, the drop in the level of the gonadotropins to less than 10 mIU/ML and an estradiol level of 23.5 pg/mL in our patient suggests negative feedback from functioning ovaries. This emphasizes the fact that some ovarian function can be maintained even in a heavily treated terminal cancer patient made possible by ovarian transposition.
We do not discount that isolated ovarian metastasis remains a risk in ovarian transposition. However, for those already with high risk of recurrence, we believe that this easily performed procedure does not alter the prognosis of the disease. Quality of life remains an important issue in cancer care. The present indications for ovarian transposition should be reevaluated, so that more premenopausal patients can benefit from this underutilized procedure.
Figures and Tables
Fig. 1
The ovaries are transposed to the antero-lateral abdominal wall, underneath the upper trocars on each side. Pelvic radiation field is below the dotted line. A, Lee-Huang point, the location of the main trocar and is midway between the umbilicus and xiphoid process; B, lower accessory trocars; O, ovaries; U, umbilicus; X, xiphoid process.
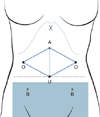
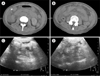
Fig. 2
Imaging survey seven months after recurrence, two years and four months after ovarian transposition. Computed tomography (CT) scan picture of right (A) and left (B) ovaries marked by metallic clips fixed at the anterior abdominal wall 3 centimeter above the umbilicus. Ultrasound pictures of right (C) and left (D) adnexae showing normal size ovaries.
ACKNOWLEDGMENTS
We would like to acknowledge the International Gynecologic Cancer Society for providing funding for the traveling scholarship for Renee Vina G. Sicam, to Chang Gung Memorial Hospital.
References
1. Morice P, Juncker L, Rey A, El-Hassan J, Haie-Meder C, Castaigne D. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil Steril. 2000. 74:743–748.
2. Landoni F, Zanagnolo V, Lovato-Diaz L, Maneo A, Rossi R, Gadducci A, et al. Ovarian metastases in early-stage cervical cancer (IA2-IIA): a multicenter retrospective study of 1965 patients (a Cooperative Task Force study). Int J Gynecol Cancer. 2007. 17:623–628.
3. Yamamoto R, Okamoto K, Yukiharu T, Kaneuchi M, Negishi H, Sakuragi N, et al. A study of risk factors for ovarian metastases in stage Ib-IIIb cervical carcinoma and analysis of ovarian function after a transposition. Gynecol Oncol. 2001. 82:312–316.
4. Nakanishi T, Wakai K, Ishikawa H, Nawa A, Suzuki Y, Nakamura S, et al. A comparison of ovarian metastasis between squamous cell carcinoma and adenocarcinoma of the uterine cervix. Gynecol Oncol. 2001. 82:504–509.
5. Morice P, Haie-Meder C, Pautier P, Lhomme C, Castaigne D. Ovarian metastasis on transposed ovary in patients treated for squamous cell carcinoma of the uterine cervix: report of two cases and surgical implications. Gynecol Oncol. 2001. 83:605–607.
6. Han SS, Kim YH, Lee SH, Kim GJ, Kim HJ, Kim JW, et al. Underuse of ovarian transposition in reproductive-aged cancer patients treated by primary or adjuvant pelvic irradiation. J Obstet Gynaecol Res. 2011. 37:825–829.
7. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990. 38:352–357.
8. Tewari KS, Monk BJ. DiSaia PJ, Creasman WT, editors. Invasive cervical cancer. Clinical gynecologic oncology. 2007. 7th ed. Philadelphia: Mosby Elsevier;51–120.
9. Steigrad S, Hacker NF, Kolb B. In vitro fertilization surrogate pregnancy in a patient who underwent radical hysterectomy followed by ovarian transposition, lower abdominal wall radiotherapy, and chemotherapy. Fertil Steril. 2005. 83:1547–1549.
10. Kovacs P, Stangel JJ, Santoro NF, Lieman H. Successful pregnancy after transient ovarian failure following treatment of symptomatic leiomyomata. Fertil Steril. 2002. 77:1292–1295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download